Zingiberaceae - syn. Zingiber aromaticum Nor., Amomum zingiber L., Amomum angustifolium Salisb.
ginger, common ginger, Canton ginger, stem ginger, Ingwer
Erect perennial from Southeast Asia, 50-100cm high; rhizomes branched, yellowish inside, thickened, fleshy, strongly aromatic; leaves sessile, lanceolate, 1-2cm wide, pointed; inflorescences arising from rhizomes; flowers yellow-green with a small dark purple or purplish-black marked lip; fruit a 3-valved, fleshy capsule; widely cultivated for medicine and spice.
http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200028468
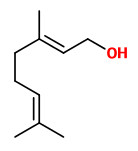
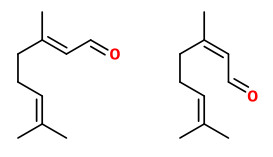
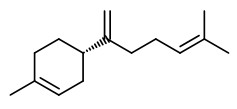
The pungent principles of ginger rhizomes could be extracted by liquid carbon dioxide and identified as [6]-gingerol (11.88% w/w extract), [8]-gingerol (1.67%), [10]-gingerol (2.38%), and [6]-shogaol (trace).
[Chen, Chu Chin, et al. „Pungent compounds of ginger (Zingiber officinale Roscoe) extracted by liquid carbon dioxide.“ Journal of Agricultural and Food Chemistry 34.3 (1986): 477-480]
„Japanese fresh ginger rhizomes (Shinshoga in Japanese) have a strong citrus-like, camphoraceous, floral musty, and fatty odor. The extract of ginger used in this experiment ([n-hexan], yield, 0.2%) posessed this fresh ginger odor. This characteristic odor was concentrated in the O-part prepared by column chromatography… 1,8-cineol, borneol, linalool, and geraniol are the important odorants in the fresh ginger odor.“
Most intensive flavor compounds (showing the highest flavor dilution factors, FD) of an extract fraction (consisting of the oxygenated hydrogens) from fresh ginger rhizomes were linalool (floral, FD 20), geraniol (floral rosy, FD 20), geranial (citrus-like, FD 17), neral (cirus-like, FD 15), citronellyl acetate (musty, FD 16), borneol (dry-camphoraceous, FD 16), 1,8-cineol (camphoraceous, FD 15), geranyl acetate (FD 14), (E)-2-octenal (green fatty, FD 12), (E)-decenal (fatty green, FD 11), and (E)-2-dodecenal (fatty green, FD 11).
[Identification of the characteristic odorants in fresh rhizomes of ginger (Zingiber officinale Roscoe) using aroma extract dilution analysis and modified multidimensional gas chromatography-mass spectroscopy., Nishimura, O., Journal of Agricultural and Food Chemistry, 43(11), 1995, 2941-2945]
Major pungent principle of ginger are gingerols, together with zingerone and shogaols, which are formed from gingerols when ginger is dried or cooked. „Ginger (Zingiber officinale Roscoe), a monocotyledonous, sterile cultigen, is widely used as a spice, flavoring agent, and herbal medicine. The pungency of fresh ginger is due to a series of homologous phenolic ketones of which [6]-gingerol is the major one. The gingerols are thermally unstable and can be converted to their corresponding shogaols, which are present in dried ginger. Fresh rhizomes of 17 clones of Australian ginger, including commercial cultivars and experimental tetraploid clones, were assayed by HPLC for gingerols and shogaols. [6]-Gingerol was identified as the major pungent phenolic compound in all samples, while [8]- and [10]-gingerol occurred in lower concentrations. One cultivar known as “Jamaican” contained the highest concentrations of all three gingerols and was the most pungent of the clones analyzed. Gingerols were stable in ethanolic solution over a 5-month period when stored at 4 °C. Shogaols were not identified in the extracts prepared from fresh rhizomes at ambient temperature, confirming that these compounds are not native constituents of fresh ginger. In contrast to previous findings, this study did not find significant differences in gingerol concentrations between the tetraploid clones and their parent diploid cultivar.“
[Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe)., Wohlmuth, H., Leach, D. N., Smith, M. K., Myers, S. P., Journal of agricultural and food chemistry, Vol.53(14), 2005, 5772-5778]
„Essential oil content was found to be 0.98 (China) and 1.58% (Thailand)… Essential oil of Thailand ginger sample contained α-pinene 3.59%, α-phallendrene 2.84%, myrcene 4.58%, β-pinene 0.74%, γ-terpinene 2.49%, 1,8-cineol 3.87%, citral 5.39% and zingiberene 30.81%. Essential oil of China ginger sample contains α-pinene 0.30%, α-phallendrene 1.02%, myrcene 4.82%, γ-terpinene 2.88%, 1,8-cineole 2.4%, α-terpinene 6.5%, citral 4.5% and zingiberene 8.0%.“
[Chemical Analysis of Essential Oil of Ginger (Zingiber officinale). Misbah Sultan , Haq Nawaz Bhatti and Zafar Iqbal. Pakistan Journal of Biological Sciences, 2005 Volume: 8 Issue: 11, 1576-1578]
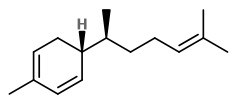
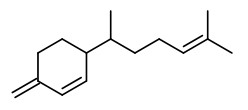
Main volatile compounds of dried ginger roots found by SDE-GC/MS were zingiberene (38.4%), β-sesquiphellandrene (18.5%), β-bisabolene (7.9%), (E,E)-farnesene (5.9%), and α-curcumene (4.7%). Enantiomeric purity (>96%ee) was shown for (S)-α-pinene and (S)-nerolidol as well as (R)-β-pinene.
[Analysis of Enantiomeric Composition of Chiral Flavor Components from Dried Ginger (Zingiber afficinale Roscoe)., Seo, H.Y., No, K.M., Shim, S.L., Ryu, K.Y., Han, K.J., Gyawali, R., Kim, K.S., Journal of the Korean Society of Food Science and Nutrition, 35(7), 2006, 874-880] http://ocean.kisti.re.kr/downfile/volume/kfn/SPOOBG/2006/v35n7/SPOOBG_2006_v35n7_874.pdf
„The essential oil counted for 1.2% of the total weight … zingiberene is the predominant compound belonging to the sesquiterpene hydrocarbons and constituted approximately 32% of the total extracted essential oil. The specific aroma of ginger is predominantly related to zingiberene… Totally seventeen compounds were identified… AR-curcumene (15%), beta-sesquiphellandrene (15%), … while the oxygenated terpenes namely endo-borneol and geraniol are present at lower concentrations and have more contributions to the flavouring characteristics of the oil.“
[Isolation and Identification of Ginger Essential Oil. Z. Kamaliroosta, L. Kamaliroosta, A. H. Elhamirad, Journal of Food Biosciences and Technology, Islamic Azad University, Science and Research Branch, 3, 73-80, 2013]
Aroma extract dilution analysis (AEDA) showed geranial, eucalyptol, β-linalool, and bornyl acetate as the most potent odorants of ginger, exhibiting the highest flavor dilution factor (FD factor) of 2187. Static headspace dilution analysis (SHDA) indicated that the predominant headspace odorants were α-pinene and eucalyptol.
[Pang, Xueli, et al. „Identification of Ginger (Zingiber officinale Roscoe) Volatiles and Localization of Aroma-Active Constituents by GC-Olfactometry.“ Journal of Agricultural and Food Chemistry 65.20 (2017): 4140-4145]
„Twenty key odorants recently identified in raw and roasted ginger were quantitated by means of stable isotope dilution assays, of which six assays were newly developed. Odor activity values (OAV; ratio of concentration to odor threshold) revealed 1,8-cineol (eucalyptus-like) with by far the highest value of 65 000 followed by myrcene (geranium-like), for which an OAV of 19 000 was calculated. In addition, (R)-citronellal, geranial, (R)-linalool, (E)-isoeugenol, and (E)-2-octenal contributed with high OAVs to the overall aroma profile of the fresh, raw ginger. An aroma recombinate prepared with 20 reference compounds in the same concentrations as determined in the ginger sample successfully matched the overall aroma profile.“
[Schaller, Tanja, and Peter Schieberle. „Quantitation of key aroma compounds in fresh, raw ginger (Zingiber officinale Roscoe) from China and roasted ginger by stable isotope dilution assays and aroma profiling by recombination experiments.“ Journal of Agricultural and Food Chemistry 68.51 (2020): 15284-15291]
„In raw ginger, the highest FD factors were found for (E)-isoeugenol, 1,8-cineol, vanillin, geranial, and linalool. After roasting, in particular, the FD factors of 3-(methylthio)propanal (cooked potato-like), 4-hydroxy-2,5-dimethyl-3(2H)-furanone (caramel-like), 3-hydroxy-4,5-dimethyl-2(5H)-furanone (seasoning-like), and geraniol were substantially increased. The application of static headspace/olfactometry (SHO) on ground raw ginger revealed a high FD factor for highly volatile acetaldehyde which clearly decreased after roasting. By contrast, the SHO application revealed high FD factors for malty smelling methylpropanal and 3-methylbutanal, which both were exclusively detected in roasted ginger. Thirteen odorants, namely, decanoic acid, (Z)-2-decenal, (Z)-4-decenal, (E)-4,5-epoxy-(E)-2-decenal, (E)-4,5-epoxy-(E)-2-undecenal, fenchol, (Z)-3-hexenal, 3-hydroxy-4,5-dimethyl-2(5H)-furanone, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, 3-methyl-2-buten-1-thiol, 2-methylpropanal, (E)-2-nonenal, and 1-nonen-3-one, were identified in ginger for the first time.“
[Schaller, Tanja, and Peter Schieberle. „Comparison of the Key Aroma Compounds in Fresh, Raw Ginger (Zingiber officinale Roscoe) from China and Roasted Ginger by Application of Aroma Extract Dilution Analysis.“ Journal of Agricultural and Food Chemistry 68.51 (2020): 15292-15300]
Several sesquiterpenes with in vitro antirhinoviral activity were isolated from dried rhizomes of Indonesian ginger, Z.officinale. The most active was β-sesquiphellandrene.
[Isolation of antirhinoviral sesquiterpenes from ginger (Zingiber officinale)., Denyer, C.V., Jackson, P., Loakes, D.M., Ellis, M.R., Young, D.A., Journal of Natural Products, 57(5), 1994, 658-662]
Tea and other preparations from rhizome are used to treat nausea, vomiting and seasickness.
[Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials., Ernst, E., Pittler, M. H., British journal of anaesthesia, Vol.84(3), 2000, 367-371] http://bja.oxfordjournals.org/content/84/3/367.full.pdf
„In randomised controlled studies, the efficacy of powdered ginger has been demonstrated in several studies most often with the following dosages and treatment durations:
1.Pregnancy-induced nausea and vomiting: 500 mg 3 times daily for 3-5 days.
2.Postoperative nausea and vomiting: 1000 mg 1 hour before induction of anaesthesia.
3.Motion-induced nausea and vomiting: 1000 mg 1 hour before start of travel.
The beneficial effect in nausea and vomiting is supported by experimental animal studies and human
pharmacodynamic studies that have shown increased gastric emptying after ingestion of ginger root,
probably caused by peripheral blocking of receptors involved in smooth muscle contraction in the
gastrointestinal tract by gingerols and shogaols contained in ginger.“
[HMPC Assessment report on Zingiber officinale Roscoe, rhizoma, 2012] http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2012/06/WC500128140.pdf
„Fresh, but not dried, ginger is effective against HRSV-induced plaque formation on airway
epithelium by blocking viral attachment and internalization… Fresh ginger dose-dependently inhibited HRSV-induced plaque formation in both HEp-2 and A549 cell lines (p<0.0001). In contrast, dried ginger didn’t show any dose-dependent inhibition.“
[Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines., San Chang, J., Wang, K.C., Yeh, C.F., Shieh, D.E., Chiang, L.C., Journal of ethnopharmacology, 145(1), 2013, 146-151]
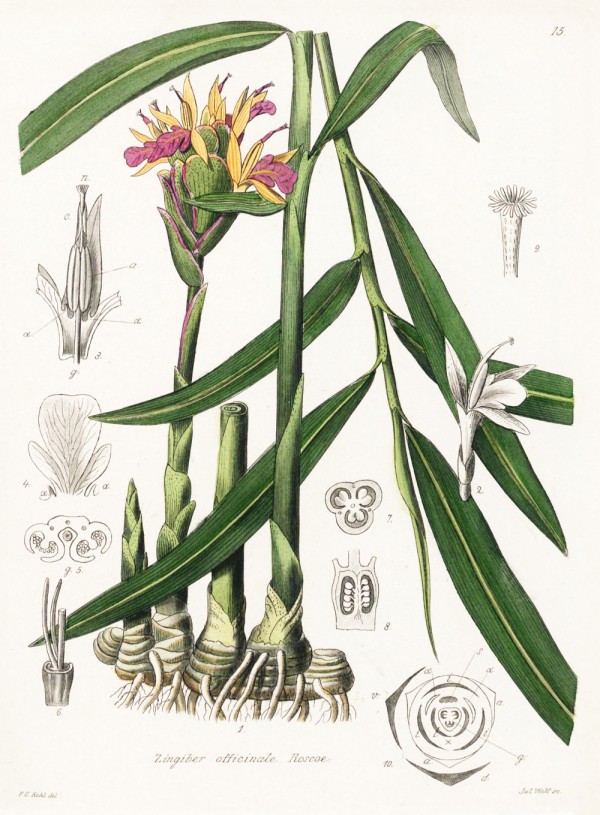
Kohl,F.G., Die officinellen Pflanzen der Pharmacopoea Germanica, t.15 (1891-1895)
http://plantgenera.org/species.php?id_species=1082674

Zingiber officinale cultivation, Mudigere 2014, efloraofindia
https://sites.google.com/site/efloraofindia/species/m---z/z/zingiberaceae/zingiber/zingiber-officinale