Paris quadrifolia L. - Trilliaceae - herb Paris, true love, (Vierblättrige) Einbeere
Perenniel woodland herb, up to 30cm high, native to Europe, Caucasus, Sibiria; rhizome creeping; flowering shoots erect, glabrous, formed every year at the apex of the rhizome; leaves usually 4, elliptic to obovate, in a whorl at the top of the stem; inflorescence a single terminal, erect, long-stalked flower; sepals lanceolate, 2-3.5cm, green; petals nearly as long as sepals, greenish yellow; fruit a poisonous globose black berry.
„Once germination has begun, emergence of seedlings takes at least 2 years, and it takes several more years for plants to progress from seedlings to become flowering plants.“
[Biological Flora of the British Isles: Paris quadrifolia L., Jacquemyn, H., Brys, R., Hutchings, M.J., Journal of ecology, 96(4), 2008, 833-844] http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2745.2008.01397.x/full
The crushed fresh herb was used externally in former times to treat wounds and ulcers, ailments of the eyes (doctrine of signatures: eye-resembling berry) and internally (rhizome) as emetic. Today the herb of P.quadrifolia is only used in homoepathic preparations to treat headache, inflammation of the upper respiratory system and problems with senses of smell and touch.
[Hagers Handbuch der Pharmazeutischen Praxis, Springer 2010]
The sterodial saponin, pennogenin-3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside showed strong cardiotoxic effects in an in vitro cellular model system.
[Chemical composition and biological activity of Paris quadrifolia L., Jenett-Siems, K., Krause, N., Siems, K., Jakupovic, S., Wallukat, G., Melzig, M.F., Zeitschrift für Naturforschung C, 67(11-12), 2012, 565-570]
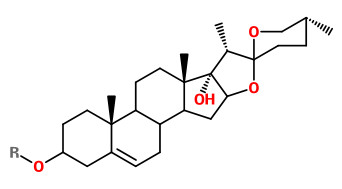 cardiotoxic/cytotoxic pennogenyl saponins (R = sugar part)
cardiotoxic/cytotoxic pennogenyl saponins (R = sugar part)
Pennogenyl saponins pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside and pennogenin 3-O-α-L-rhamnopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→4)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside exhibited strong cytotoxic activity against HL-60, HeLa and MCF-7 tumour cells.
[Isolation and identification of cytotoxic compounds from the rhizomes of Paris quadrifolia L., Gajdus, J., Kaczyński, Z., Kawiak, A., Łojkowska, E., Stefanowicz-Hajduk, J., Ochocka, J.R., Stepnowski, P., Pharmacognosy magazine, 10(Suppl 2), 2014, S324]
Regarding the the molecular mechanisms of cytotoxic effects of pennogenyl saponins, both the extrinsic death receptor and intrinsic mitochondrial pathways are involved in the programmed cell death.
[Pennogenyl Saponins from Paris quadrifolia L. Induce Extrinsic and Intrinsic Pathway of Apoptosis in Human Cervical Cancer HeLa Cells., Stefanowicz-Hajduk, J., Bartoszewski, R., Bartoszewska, S., Kochan, K., Adamska, A., Kosiński, I., Ochocka, J.R., PloS one, 10(8), 2015, e0135993] http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0135993

Redouté, P.J., Les Liliacées, vol.4 t.226 (1805-1816)
http://plantgenera.org/species.php?id_species=749983
