Leptospermum scoparium Forster & Forster - Myrtaceae - manuka, New Zealand tea tree, Manuka, Südseemyrte
Shrub or tree, up to 4m high, native to New Zealand and southeast Australia; leaves narrow-lanceolate or ovate, coriaceous, rigid, acute, pungent; flowers axillary, or occasionally terminal on branchlets, ± sessile, usually solitary white, rarely pink petals.
http://www.nzflora.info/factsheet/Taxon/Leptospermum_scoparium.html
„Mānuka sawdust imparts a delicious flavour when used for smoking meats and fish. It is cultivated in New Zealand for mānuka honey, produced when honeybees gather the nectar from its flowers, and for the pharmaceutical industry.“ wikipedia
Manuka oil is the essential oil produced by steam distillation of the leaves (with twigs). Major components of commercial New Zealand essential oils are triketones (flavesone 4%, iso-leptospermone 4%, leptospermone 15%) and sesquiterpene hydrocarbons (≥60%), including calamenene (14%), α-cubebene (4%), α-copaene (5%), δ-cadinene (6%), cadina-1,4-diene (5%) and cadina-3,5-diene (4%), δ-amorphene (3%), aromadendrene (2%) and β-caryophyllene (2%).
[Chemical, physical and antimicrobial properties of essential oils of Leptospermum scoparium and Kunzea ericoides. Porter, N. G., & Wilkins, A. L., Phytochemistry, Vol.50(3), 1999, 407-415]
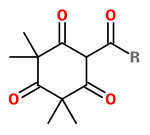 flavesone R=2-propyl isoleptospermone R=2-butyl leptospermon R=2-methylprop-1-yl | 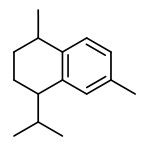 calamenene (herbal) | 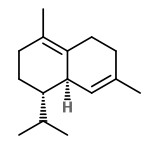 δ-cadinene (herbal-thyme) | 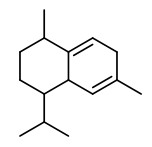 cadina-1,4-diene (spicy-fruity) | 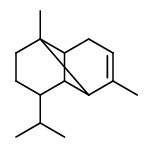 α-copaene (woody) |
Among five tea tree oils (Australian tea tree, cajupute, niaouli, kanuka and manuka), manuka oil showed highest effectiveness against gram-positive bacteria and dermatophytes, but was ineffective against Candida albicans and moulds. It tourned out that the β-triketone complex (flavesone, iso-leptospermone, and leptospermone) is the active principle.
[A comparative study of the in vitro antimicrobial activity of tea tree oils sl with special reference to the activity of beta-triketones., Christoph, F., Kaulfers, P.M., Stahl-Biskup, E., Planta medica, 66(6), 2000, 556-560]
„The triketone chemotype of manuka, Leptospermum scoparium (Myrtaceae), is commercially important because of its antimicrobial activity. Oils from 36 individual plants on the East Cape of New Zealand all showed similar high triketone contents (>20% total triketones) with little seasonal variation. Analyses of oils from 261 individual manuka plants collected from 87 sites throughout New Zealand showed that the high triketone chemotype was localised on the East Cape, although oils with triketone levels up to 20% were found in the Marlborough Sounds area of the South Island. Cluster analysis revealed other chemotypes localised on other areas. Ten further chemotypes are described: α-pinene; sesquiterpene-rich with high myrcene; sesquiterpene-rich with elevated caryophyllene and humulene; sesquiterpene-rich with an unidentified sesquiterpene hydrocarbon; high geranyl acetate; sesquiterpene-rich with high γ-ylangene + α-copaene and elevated triketones; sesquiterpene-rich with no distinctive components; sesquiterpene-rich with high trans-methyl cinnamate; high linalol; and sesquiterpene-rich with elevated elemene and selinene. Some of the chemotypes contained aroma compounds at relatively high levels, with a geranyl acetate-rich oil being most notable.“
[Douglas, Malcolm H., et al. „Essential oils from New Zealand manuka: triketone and other chemotypes of Leptospermum scoparium.“ Phytochemistry 65.9 (2004): 1255-1264]
Commercial Manuka oils contained β-triketones (flavesone 3-8%, iso-leptospermone 2-7%, and leptospermone 10-20%) and sesquiterpenes like α-cubebene (2-7%), α-copaene (2.5-7.5%), β-caryophyllene (2-3%), α-selinene (2-7%), β-selinene (1-6%), tr-calamenene (9-18%), δ-cadinene (3-8%), cadina-1,4-diene (3-8%), cadina-3,5-diene (2-12%), and δ-amorphene (2-6%).
[Wolz, Dietmar, and Gerhard Buchbauer. Aromatherapie in Wissenschaft und Praxis. Ed. Wolfgang Steflitsch. Stadelmann, 2013, 591-593]
Manuka honey, a monofloral honey produced in Australia and New Zealand from the nectar of the mānuka tree, is commonly sold as an alternative medicine. Manuka honey is known for its exceptional antibacterial activity, which is due to high amounts of the 1,2-dicarbonyl compound methylglyoxal (MGO), which is formed via non-enzymatic dehydration from dihydroxyacetone (DHA) during honey maturation.
„MGO and DHA are highly reactive substances, leading to a variety of unique chemical reactions. During Strecker reaction between proline and MGO, 2-acetyl-1-pyrroline (2-AP), an important aroma compound, is formed. Using liquid–liquid extraction and gas chromatography–mass spectrometry analysis, 2-AP was identified unambiguously in manuka honey for the first time. Quantitation was carried out via external matrix calibration, using a synthetic 2-AP standard and artificial honey. The 2-AP concentration in 11 commercial samples of manuka honey ranged from 0.08 to 0.45 mg/kg. For manuka honey samples containing MGO in concentrations above 250 mg/kg, significantly higher amounts of 2-AP were found when compared to non-manuka honeys. When high amounts of MGO were artificially added to non-manuka multifloral honey, an increase of the 2-AP concentration from 0.07 to 0.40 mg/kg after 12 weeks of storage at 37 °C was observed, concomitant with a significant increase in the concentration of 5-hydroxymethylfurfural (HMF). No increase of 2-AP was found during storage at ambient temperature. 2-AP together with MGO can be a suitable parameter for the quality control of manuka honey.“
[Rückriemen, Jana, et al. „Identification and quantitation of 2-acetyl-1-pyrroline in manuka honey (Leptospermum Scoparium).“ Journal of Agricultural and Food Chemistry 63(38), 2015, 8488-8492]

The botanist’s repository [H.C. Andrews], vol.10 t.622 (1810-1812) [H.C.Andrews] plantgenera.org

Leptospermum scoparium, Te Oka, New Zealand (2025) © Nathan Campbell CC BY-SA 4.0 inaturalist.org
