Citrus aurantiifolia (Christm.) Swingle - syn. Citrus acida Roxb.; Citrus lima Lunan; Limonia aurantiifolia Christm. - Rutaceae - Mexican lime, Key lime, Echte Limette, Mexikanische Limette, Saure Limette
[C. medica × C. micrantha] USDA NPGS
„C. aurantiifolia is a shrubby tree, to 5 m (16 ft), with many thorns… The leaves are ovate, 2.5-9 cm (1-3 1/2 in) long, resembling orange leaves (the scientific name aurantiifolia refers to this resemblance to the leaves of the orange, Citrus aurantium).“ wikipedia
The fruit (2.5-5cm in diameter - smaller than Persian lime) is green when immature and pale yellow when mature.
„The Mexican lime is native to the Indo-Malayan region. It was unknown in Europe before the Crusades and it is assumed to have been carried to North Africa and the Near East by Arabs and taken by Crusaders from Palestine to Mediterranean Europe. In the mid-13th Century, it was cultivated and well-known in Italy and probably also in France. It was undoubtedly introduced into the Caribbean islands and Mexico by the Spaniards, for it was reportedly commonly grown in Haiti in 1520. It readily became naturalized in the West Indies and Mexico…
The Mexican lime, because of its special bouquet and unique flavor, is ideal for serving in half as a garnish and flavoring for fish and meats, for adding zest to cold drinks, and for making limeade… The hand-pressed peel oil is mainly utilized in the perfume industry.“
Morton, J. 1987. Mexican Lime. p. 168-172. In: Fruits of warm climates. Julia F. Morton, Miami, FL.
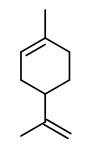
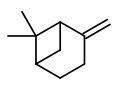
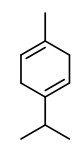
„Distilled lime oil is unique among the citrus oils because it is a reaction flavor resulting from acid-catalyzed transformations, at elevated temperature, of principally the terpenes found in lime peel and fruit… Typical distillation conditions are 8-10h at pH<3 and 96-98°C. Distilled oil counts for 95% or more of lime oil production… The major reactions are the the acid-catalyzed hydrolysis and rearrangement reactions of the bicyclic hydrocarbons: α- and ß-pinene, sabinene, and α-thujene. The major products of these reactions are well known: alcohols, α-terpineol, terpinen-4-ol, α-fenchol, borneol, isoborneol; and the hydrocarbons terpinolene, limonene, fenchene, camphene, γ-terpinene, α-terpinene.“
[Chamblee, Theresa S., and Benjamin C. Clark Jr. „Analysis and chemistry of distilled lime oil (Citrus aurantifolia Swingle).“ Journal of Essential Oil Research 9.3 (1997): 267-274]
„In lime peel oils, four chemotypes were distinguished: limonene; limonene/γ-terpinene; limonene/β-pinene/γ-terpinene; and limonene/γ-terpinene/β-pinene/oxygenated products.“
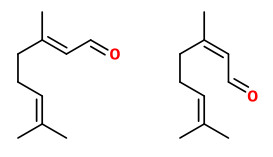
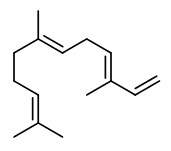
Components were e.g. limonene (40-94%), ß-pinene (0-19%), sabinene (0.1-19.6%), γ-terpinene (0-21%), p-cymene (tr-5.3%), citronellal (tr-1.1%), neral (0-2.3%), geranial (0-4.0%), neryl acetate (0-2.0%), geranyl acetate (0-2.4%), (E)-α-bergamotene (0.2-1.6%), ß-bisabolene (0.3-2.4%), and (E,E)-α-farnesene (0-1.9%).
[Lota, Marie-Laure, et al. „Volatile components of peel and leaf oils of lemon and lime species.“ Journal of Agricultural and Food Chemistry 50.4 (2002): 796-805]
Main components of the solvent extracted peel oil (dichloromethane, yield 0.4%) were limonene (30.5%), γ-terpinene (19.2%), ß-pinene (7.9%), 5,7-dimethoxycoumarin (6.6%), geranial (5.9%), neral (3.8%), 7-methoxycoumarin (3.3%), bergaptene (2.9%), ß-bisabolene (2.8%), neryl acetate (2.2%), α-bergamotene (1.7%), and (E,E)-α-farnesene (1.4%).
[Craske, John D., Norman Suryadi, and Michael Wootton. „A comparison of the peel oil components of Australian native lime (Microcitrus australe) and Mexican lime (Citrus aurantifolia Swingle).“ Journal of the Science of Food and Agriculture 85.3 (2005): 522-525]
Distilled lime oils, prepared by steam distilliation of the whole fruits, contain a certain amount of products formed by acid-catalyzed reactions like camphene, α,p-dimethylstyrene, α-fenchol, p-cymene, α-terpineol, ß-terpineol, terpinen-4-ol, terpinen-1-ol, borneol, 1,4-cineole, and 1,8-cineole. This leads to a more terpenic odour, which is desired in perfumery. Among the citrus oils, lime oil is the richest in containing farnesenes formed during distillation by dehydration of nerolidol and farnesol.
The more expensive cold-pressed oils tend organoleptically towards lemon oil, as they contain citral and decanal, which are not present in distilled oils.
[Scent and Chemistry, Günther Ohloff, Wilhelm Pickenhagen, Philip Kraft, Wiley-VCH, 2012, 233-234]
Cold-pressed peel oils are characterized by monoterpenes like (+)-limonene (50-61%), ß-pinene (9-15%), and γ-terpinene (11-16%), together with oxygenated terpenoids like neral (0.5-1.6%), geranial (1.2-3.1%), neryl acetate (0.3-2.9%), geranyl acetate (0.2-0.5%), and sesquiterpenoids like (E)-α-bergamotene (0.7-1.5%), ß-bisabolene (1.5-2.4%), and (Z)-α-farnesene (0-0.3%). They may also contain up to 3% of the photo-sensitizing furocoumarines.
Distilled peel oils are characterized by monoterpenoids like (+)-limonene (41-49%), α-terpinene (2.6-4.0%), γ-terpinene (9-14%), p-cymene (2.5-4.0%), terpinolene (7.3-9.4%), α-terpineol (2.6-4.0%), and 1,8-cineole (1.5-2.5%), together with minor amounts of sesquiterpenoids like (E)-α-bergamotene (0.5-2.5%), ß-bisabolene (1.0-2.6%), and (Z)-α-farnesene (0.4-1.7%).
[Wolz, Dietmar, and Gerhard Buchbauer. Aromatherapie in Wissenschaft und Praxis. Ed. Wolfgang Steflitsch. Stadelmann, 2013, 574-577]

Citrus aurantiifolia, Thailand (2024) © kanapol99 CC BY-SA 4.0 inaturalist.org

Citrus aurantiifolia (Christm.) Swingle as Citrus medica L. var.acida
Curtis’s Botanical Magazine, t. 6731-6792, vol.110 [ser.3, vol.40] t.6745 (1884) [M.Smith] botanicalillustrations.org