Angelica archangelica L. - syn.Angelica officinalis Moench; Angelica sativa Mill.; Archangelica officinalis (Moench) Hoffm. - Apiaceae
angelica, garden angelica, European angelica, wild parsnip, (Echte) Engelwurz, (Arznei-)Angelika, Brustwurz
Stout perennial 1.20-2.50m tall, native to noerthern Europe and Northwest Asia; leaves biternate, the lower leaves sometimes 30-90 cm long; segments oval, unevenly toothed or cut, terminal segments usually 3-lobed; umbels 8-15 cm across, with 20-40 rays; umbellets subtended by many linear bractlets.
A.archangelica extracts are used extensively in the liquor industry as a flavoring in liquor such as boonekamp, benedictine, and chartreuse. http://de.wikipedia.org/wiki/Arznei-Engelwurz
„Angelica root is nowadays specifically recommended for the treatment of appetite loss, stomach cramps and flatulence… The pharmacology appears to be poorly known but the plant stimulates the flow of gastric juices and has definite antispasmodic and cholagogue activities.“
[Medicinal Plants of the World. Ben-Erik Van Wyk and Michael Wink, Pretoria 2004, 48]
„The volatile components isolated from the root of two wild angelica strains (Angelica archangelica L. var. Archangelica) grown in the northern Finland were compared with the garden angelica (var. Sativa) grown in the north and in the south of Finland. More than 80 compounds were determined in the Soxhlet extracts by gas chromatography, and 67 were identified by gas chromatographymass spectrometry. Large variability in the relative amounts of the compounds was found. β-Phellandrene was the main component in var. Archangelica and sabinene in var. Sativa. The relative proportion of both hydrocarbon monoterpenes and oxygenated monoterpenes was larger in var. Sativa cultivated in the north than in the south of Finland“
[Characterization of volatile composition and odor of angelica (Angelica archangelica subsp. archangelica L.) root extracts. Kerrola, K., Galambosi, B., Kallio, H., Journal of Agricultural and Food Chemistry, 42(9), 1994, 1979-1988]
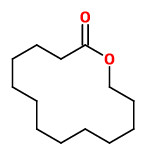
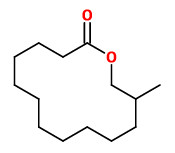
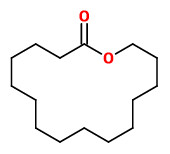
Angelica root oil contains a small fraction of musk-smelling macrolides. In this fraction the ω-alkanolides C13 (50.0%), C14 (0.5%), C15 (42.0%, Exaltolide®), C16 (trace), and C17 (3-5%) as well as 12-methyl-13-tridecanolide (4.0%) were identified. The enantiomeric composition was found to be 72.4 % (12R)-(+)-12-methyl-13-tridecanolide (musky, sandalwood, pear) and 27.6 % (12S)-(-)-12-methyl-13-tridecanolide (musky, animalic, camphor).
[Characterization of the Macrolide Fraction of Angelica Root Oil and Enantiomeric Composition of 12-Methyl-13-tridecanolide. Schultz, K., Kraft, P., Journal of Essential Oil Research, Vol.9(5), 1997, 509-514]
„Roots and seeds of Angelica archangelica L. were collected from different localities in western, eastern and northern Finnish Lapland. Two root samples and 33 seed samples were extracted with n-hexane and analysed by GC-MS using a chiral β-cyclodextrin phase. Major compounds in the root oils were (−)-α-pinene (19-42%) and (+)-sabinene (21-28%). One of the oils contained 22% (+)-3-carene but the other one had none at all. The seed oils were dominated by (−)-β-phellandrene (>60%). Other major compounds were (+)-sabinene, (+)-α-pinene, myrcene, (−)-α-phellandrene, (−)-α-pinene and (−)-limonene. Some statistically significant differences between seed samples from different localities could also be found.“
[Enantiomeric composition of monoterpene hydrocarbons in n‐hexane extracts of Angelica archangelica L. roots and seeds.Holm, Y., Vuorela, P., Hiltunen, R., Flavour and fragrance journal, Vol.12(6), 1997, 397-400]
Main component of the pale yellow essential oil of the seeds (1.13%; strong peppery odor) was β‐phellandrene (74.7%). Other components were e.g. α-phellandrene, α-pinene, and linalool. A small amount of macrocyclic lactones was found in the seed oil including tridecano‐13‐lactone (0.3%), pentadecano‐15‐lactone (0.2%), and heptadecano‐17‐lactone (trace).
The essential oil of the roots contained a larger amount of macrocyclic lactones (1.3%) such as tridecano‐13‐lactone (0.65%), 12‐methyltridecano‐13‐lactone (0.06%), tetradecano‐14‐lactone (trace), pentadecano‐15‐lactone (0.53%), 14‐methylpentadecano‐15‐lactone (trace), hexadecano‐16‐lactone (trace), and heptadecano‐17‐lactone (0.06%). Main components of the root oil were β‐phellandrene (26.6%), α-phellandrene (19.1%), and α-pinene (15.7%).
[Lopes, Daíse, Herbert Strobl, and Paul Kolodziejczyk. „14‐Methylpentadecano‐15‐lactone (Muscolide): A New Macrocyclic Lactone from the Oil of Angelica archangelica L.“ Chemistry & biodiversity 1.12 (2004): 1880-1887]
see also [Lopes, Daíse, Herbert Strobl, and Paul Kolodziejczyk. „14‐Methylpentadecano‐15‐lactone (Muscolide): A New Macrocyclic Lactone from the Oil of Angelica archangelica L.“ Perspectives in flavor and fragrance research. Ed. Karl AD Swift. Helvetica Chimica Acta, 2005, 47-53]
„(3E,5Z)-undeca-1,3,5-triene also occurs in olfactorly significant amounts in, for example, angelica root oil, lavender oil, celery herb oil, the scent of fresh pineapple, mandarin, peppermint, and many Coryanthes species.“
[Meaningful Scents around the World, R.Kaiser, 2006, 58; R.Kaiser internal Givaudan files]
Imperatorin is the most abundant furanocoumarin in the ethanolic seed extract of A.archangelica, comprising 40-50% of its total furanocoumarin content. Xanthotoxin is the second most abundant in the reaction mixture in the assay of the seed extract. The Acetylcholinesterase activity of imperatorin and xanthotoxin from
A. archangelica was measured, and xanthotoxin proved much more potent than imperatorin… However, furanocoumarins seem to have a minor part in the total activity of this extract. Synergistic interaction was
observed between the extracts of A. archangelica and G. sylvaticum.
[Inhibition of acetylcholinesterase by extracts and constituents from Angelica archangelica and Geranium sylvaticum. Sigurdsson, S., Gudbjarnason, S., Zeitschrift fur Naturforschung C-Journal of Biosciences, 62(9-10), 2007, 689-693] http://www.znaturforsch.com/ac/v62c/s62c0689.pdf?origin=publication_detail
„Essential oils obtained from Angelica archangelica L. roots immediately and after storage about 2.5 months after crushing were analyzed by GC and GC MS. The dominant component in the oils from roots crushed before analysis was α-pinene (15.7-19.4%), the other major compounds being δ -3-carene (15.4-16.0%) and limonene (8.1-8.8 %) or β-phellandrene (15.4%) and δ-3-carene (14.2%), depending on the growing localities. The dominating constituent of the crushed and stored root oils was 15-pentadecanolide (7.2-14.9%). The other main constituents were coumarine osthol (5.3-8.8%) and macrocyclic lactone 13-tridecanolide (5.4-6.1%). A large part (about 70%) of monoterpene hydrocarbons was evaporated during storage from small particles of roots, while the fraction of macrocyclic lactones (14.3-25.2%) and the amounts of coumarin osthol (5.3-8.8%) increased relatively 2-5 times.“
[Changes in the chemical composition of essential oil of Angelica archangelica L. roots during storage. Nivinskienë, O., Butkienë, R., Mockutë, D., Chemija (Vilnius), 14(1), 2003, 52-56]
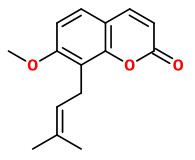 osthole (7-methoxy-8-(3-methylbut-2-enyl)-2-chromenone)
osthole (7-methoxy-8-(3-methylbut-2-enyl)-2-chromenone)
„The roots of Angelica archangelica L. were collected in three habitats (12 samples) in 1995-2002. The oils were analyzed by GC and GC/MS. The dominant component was α-pinene (15.7-20.8%) for two localities. Other three main constituents were δ-3-carene (15.4-16.9%), limonene (8.0-9.2%), sabinene (5.0-7.5%) for the first locality, and δ-phellandrene (13.5-15.4%), δ-3-carene (13.2-14.2%) and α-phellandrene (8.0-9.1%) for the second locality. The dominant oil components in the third locality were β-phellandrene (13.8-18.5%) together with α-pinene (11.4-15.0%), δ-3-carene (10.8-11.9%), α-cymene (6.8-10.6%) and α-phellandrene (5.9-8.6%). The oils contained 67.3-79.9% monoterpenoids (monoterpene hydrocarbons made up 60.2-72.6%), 9.6-19.4% sesquiterpenoids, 3.9-6.3% macrocyclic lactones and 1.2-5.3% coumarin osthol. Identified compounds (81 from 96) made up 91.4-99.2% of the oils.“
[The chemical composition of the essential oil of Angelica archangelica L. roots growing wild in Lithuania. Nivinskienė, O., Butkienė, R., Mockutė, D., Journal of essential oil research, Vol.17(4), 2005, 373-377]
Whereas 12-methyl-13-tridecanolide smells musky, the parent 14-membered macrolide tridecano-13-lactone (tridecanolide) does not. Moreover, also the enantiomers (which occure together in Angelica root oil with and R/S ratio of 72:28) „…differ significantly in their odor character: while the (+)-(12R)-isomer posesses a strong clean musk odor with sandalwood-like and fruity aspects, the enantiomer (-)-(12S) emanates an animalic musk odor with camphoraceous aspects.“
[Kraft, Philip. Perspectives in flavor and fragrance research. John Wiley & Sons, 2005, 132-133]
„The largest part of the essential oil from A. archangelica L. roots was composed of monoterpene hydrocarbons. α-pinene was found as a dominant constituent in more than half of the investigated plant oils obtained from Finland, Norway, France, and Brazil. Other dominant components such as β-phellandrene, δ-3-carene, and
β-pinene, limonene, p-cymene and α-phellandrene were also found in the A. archangelica oils. The majority of essential components we found in A. archangelica root oil corroborated the reports above and were determined to be monoterpene hydrocarbons: α-pinene (24.5%), δ-3-carene (13.8%), β-phellandrene (10.1%), p-cymene (8.8%), limonene (8.4%) and sabinene (6.3%). Forty-five compounds were identified and comprised 92.4%
of the total oil. The importance and usefulness of GC/MS chemical fingerprinting during this study demonstrated that one sample of A. sinensis was misidentified as A.archangelica by the vendor in China. Our GC/MS profile indicated that the A.sinensis oil was rich in phthalides not in monoterpene hydrocarbons and was misidentified and was not A.archangelica as originally indicated“
[Bioactivity-guided fractionation and GC/MS fingerprinting of Angelica sinensis and Angelica archangelica root components for antifungal and mosquito deterrent activity. Wedge, D. E., Klun, J. A., Tabanca, N., Demirci, B., Ozek, T., Baser, K. H. C., Zhang, J., Journal of agricultural and food chemistry, 57(2), 2009, 464-470] http://www.afpmb.org/sites/default/files/pubs/dwfp/publications/FY09/Wedge/Wedge2009.pdf
Osthole may be (partly) responsible for the antispasmodic effect of A.archangelica, as it is also responsible for the relaxant effect on the rat ileum as a component of Prangos ferulacea.
[Antispasmodic effects of Prangos ferulacea acetone extract and its main component osthole on ileum contraction., Sadraei, H., Shokoohinia, Y., Sajjadi, S.E., Mozafari, M., Research in pharmaceutical sciences, Vol.8(2), 2013, 137]
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764677/
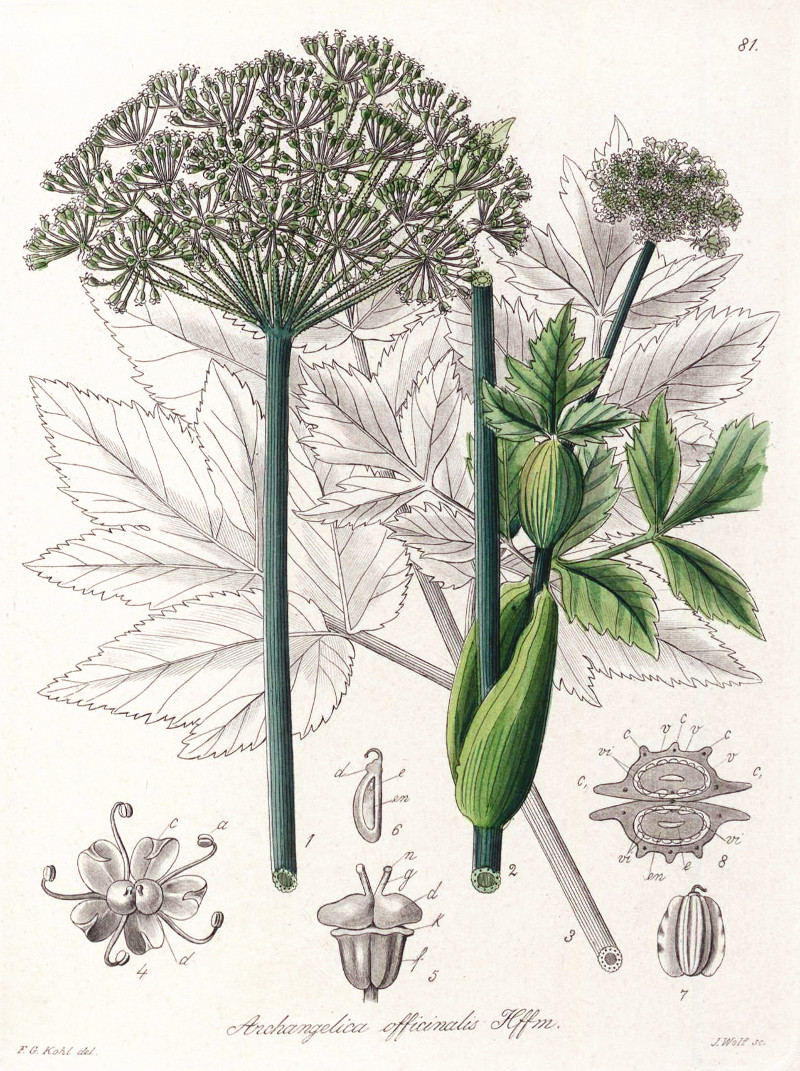
Kohl,F.G., Die officinellen Pflanzen der Pharmacopoea Germanica, t.81 (1891-1895)
http://www.plantillustrations.org/species.php?id_species=63392

Angelica archangelica, CC BY-SA 3.0, Author: Andreas Kraska