Dies ist eine alte Version des Dokuments!
Toona sinensis (A.Juss.) M.Roem. - syn.Cedrela sinensis A.Juss. - Meliaceae
香椿 xiang chun (chin.), red toon, Chinese mahogany, Chinese toon, Surenbaum
Deciduous tree, up to 40m (trunk 20m) tall, native to Southeast Asia (China to Malaysia) and India, cultivated as ornamental; „…inner bark pink to red, fibrous; sap-wood cream-colored to red, fibrous, smelling strongly of garlic and pepper when cut…
The timber is used for furniture and sieve hoop-making, and in bridge construction. The leaves are used as a vegetable in China and Malaysia, and as animal fodder in India. The trees are widely used medicinally, with the bark being used as an astringent and depurative, powdered root as a refreshment and a diuretic, and tender leaves as a carminative.“
http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200012513
„The young leaves of T. sinensis (xiāngchūn) are extensively used as a vegetable in China; they have a floral, yet onion-like flavor, attributed to volatile organosulfur compounds.“ https://en.wikipedia.org/wiki/Toona_sinensis
The volatiles of the leaves of T.sinensis were analyzed with MAE-HS-SPME/GC-MS, and 45 compounds were identified (96% of the total chromatographic area). Trans-caryophyllene (21.4%), 2-hexenal (14.6%), 5-ethylthiazole (7.5%), 2,5-dimethylthiophen (4.2%), 1-methylimidazole-2-thione (3.6%), limonene (3.5%), β-eudesmol (2.6%), 1,7,7-trimethyl-2-vinylbicyclo[2.2.1]hept-2-ene (2.6%), 2-carene (2.3%), α-caryophyllene (2.1%) and β-elemene (1.9%) were the main components. Several kinds of low concentration terpenes and sulfur compounds like β-cedrene (1.5%), α-guaiaene (1.3%) and mintsulfide (0.2%) were also present.
[Rapid Determination of Volatile Compounds in Toona sinensis (A. Juss.) Roem. by MAE-HS-SPME Followed by GC-MS., Mu, R., Wang, X., Liu, S., Yuan, X., Wang, S., Fan, Z., Chromatographia, 65(7-8), 2007, 463-467]
T. sinensis was found to contain norcysteine-containing metabolites (S,S)-γ-glutamyl-(cis-S-1-propenyl)thioglycine, (S,S)-γ-glutamyl-(trans-S-1-propenyl)thioglycine, and γ-glutamyl-(cis-S-1-propenyl)-cysteine. „They may release thiols via cleavage of the amide bond by proteases, followed by spontaneous decomposition of the resulting unstable alk(en)yl norcysteine moiety.“

[Identification of (S, S)-γ-Glutamyl-(cis-S-1-propenyl) thioglycine, a Naturally Occurring Norcysteine Derivative, from the Chinese Vegetable Toona sinensis., Li, J.X., Eidman, K., Gan, X.W., Haefliger, O.P., Carroll, P.J., Pika, J., Journal of agricultural and food chemistry, 61(31), 2013, 7470-7476]
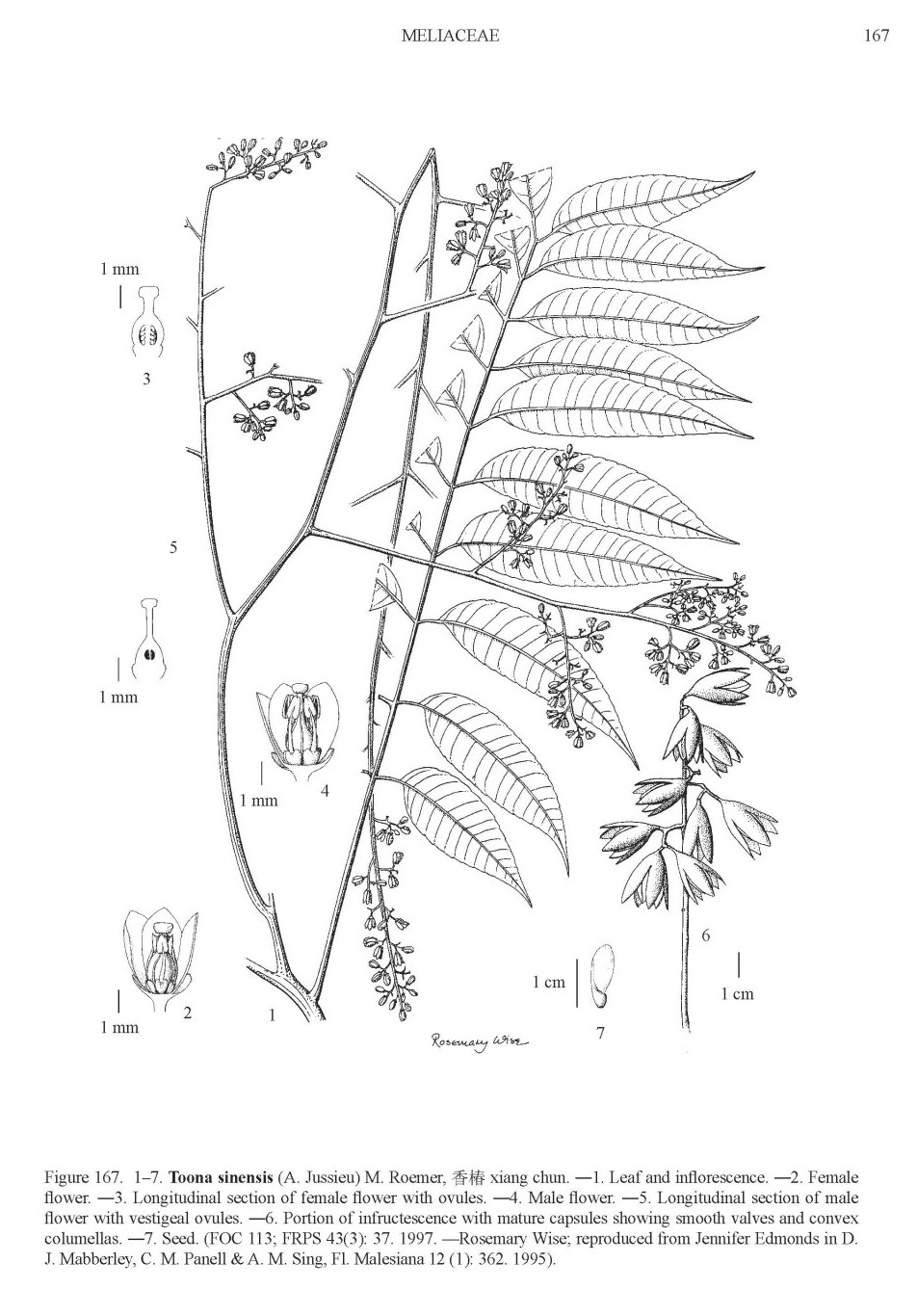
picture source: Flora of China, Harvard, http://www.efloras.org/object_page.aspx?object_id=109710&flora_id=2
