Dies ist eine alte Version des Dokuments!
Pyrus communis L. - Rosaceae - European pear, common pear, Birne
Deciduous tree, up to 20m high, native to Europe, West and Central Asia, cultivated and naturalized there.
Pyrus communis L. subsp. pyraster (L.) Ehrh. (wild pear, Wilder Birnbaum)
Pyrus communis L. subsp. communis (cultivated)
Main cultivars are: Williams pear (Bartlett pear, Williams Christ); Beurré d'Anjou; Bosc pear (Don Bosco)
„Although they point to finds of pears in sites in Neolithic and Bronze Age European sites, „reliable information on pear cultivation first appears in the works of the Greek and the Roman writers… For best and most consistent quality, European pears are picked when the fruit matures, but before they are ripe. Fruit allowed to ripen on the tree often drops before it can be picked, and in any event will be hard to pick without bruising. Pears store (and ship) well in their mature but unripe state if kept cold, and can be ripened later, a process called bletting. Some varieties, such as Beurre d'Anjou, ripen only with exposure to cold.“
http://en.wikipedia.org/wiki/European_Pear
„Eight pear varieties including ‘Bartlett’, ‘Packham's Triumph’, ‘Anjou’, ‘Comice’, ‘Bosc’, ‘Seckel’, ‘Vicar of Winkfield’ and ‘Forelle’ were ripened optimally and volatile compounds from dynamic headspace were collected and identified by GC/MS. Among 112 compounds identified from all varieties, 47 compounds are reported the first time in pears. The volatile profiles of these eight varieties are characterized by compounds in groups of esters, alcohols, hydrocarbons, aldehydes, and ketones. Esters of short to medium chain alcohols and their corresponding acids with or without some degree of unsaturation are always present as a major group of compounds in all varieties. Esters comprise 60 to 98% of total volatiles among the varieties. Hexyl acetate represents the major ester in all cultivars except ‘Bosc’ and ‘Vicar of Winkfield’ which produced butyl acetate as a major ester, and ‘Seckel’ produced ethyl 2,4 decadienoate as the major ester. Decadienoate esters were detected in all varieties, but varied in amount and isomerization characteristics. Straight-chain alcohols with 2 to 8 carbons are present as the second largest group in the profile. Two isomeric forms (E,E and E,Z) of the sesquiterpene hydrocarbon, α-farnesene, are always present in the profiles but vary considerably in amount. A small number of aldehydes and ketones are also found in some varieties but present in very small amounts.
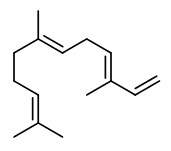 (E,E)-α-farnesene (woody green) | 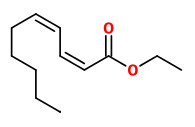 ethyl-(2Z,4Z)-decadienoate (pear fruity) | 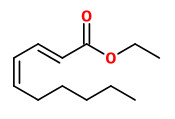 ethyl-(2E,4Z)-decadienoate (pear green) | 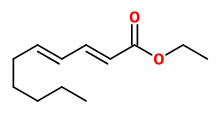 ethyl-(2E,4E)-decadienoate (fermented) |
…aroma description and intensity of the odor active compounds isolated from headspace of 'Bartlett' pears…
Among the complex mixture of approximately 64 compounds separated on the GC column, 31 compounds were odor-active. Eleven compounds clearly possessed fruity or estery aroma. Specific pear aroma was obtained from
7 different compounds including 2-methylpropyl acetate, butyl butanoate, pentyl acetate, ethyl hexanoate, hexyl acetate, ethyl cis-2,cis-4 decadienoate and ethyl trans-2,cis-4 decadienoate. The highest intensity of pear-like aroma was observed from pentyl acetate and hexyl acetate. Those two compounds were rated to have a
strong to very strong pear and fruity odor. Three isomers of ethyl decadienoates are found to possess different odor characteristics. The cis-2,cis-4 isomer provided pear-fruity scent and the trans-2,cis-4 provided pear-peel-green while the trans-2,trans-4 isomer provided alcoholic or fermented-like characteristic. Note that all of these three compounds would give similar responses if not resolved by HPLC, such as used by Quamme's group. Besides the eleven compounds which possessed pear or fruity aroma, another 7 compounds were found to have positive odor characteristics by providing floral, sweet, candy or perfume aroma. Those compounds
were in a group of esters such as propyl acetate, isoamyl acetate, 1-methylpropyl butanoate and 3-methylbutyl
3-methylbutanoate. The odor intensity of those compounds ranged from just detectable to slight on an ordinal score. Another 3 compounds in the group of esters were found to possess mushroom-like odor characteristics. These compounds include 4-hexen-1-ol acetate, 2-methylpropyl hexanoate and ethyl-2,4-octadienoate.
'Anjou' volatiles are similar to 'Packham's Triumph' pear in that they lack C3 esters and have a high degree of unsaturated acid esters. The major esters are hexyl acetate (49.2%), butyl acetate (22.1%) and hexyl hexanoate (2.6%). Surprisingly, the 2,4 decadienoate esters were found to be as high as observed in 'Bartlett' pear (3.9%) despite the general observation that Anjou pears lack typical 'Bartlett' flavor. Quamme (1984) failed to detect decadienoate esters from 'Anjou' pears by HPLC and therefore did not classify this pear as among the Bartlett-like varieties. A considerably higher content of α-farnesene was also observed (3.4%). Scald incidence has been reported to be higher in this pear compared to 'Packham's Triumph' and 'Bartlett' pear (Zoffoli, 1994). The only aldehyde, hexanal, was found in very small amount. Aldehyde compounds have been previously isolated from pear juice concentrate (Gasco, 1969) but not from the fruits.“
[Suwanagul, A., & Richardson, D. G. (1997, January). Identification of headspace volatile compounds from different pear (Pyrus communis L.) varieties. In VII International Symposium on Pear Growing 475 (pp. 605-624)]
Principal head-space volatiles from ripe pear fruit were butyl acetate, hexyl acetate, (Z)-3-hexenyl acetate, (E)-β-ocimene and the ethyl and methyl esters of (2E,4Z)-2,4-decadienoic acid. „In comparison with the known CM neonate attractant, (E,E)-α-farnesene, ethyl (2E,4Z)-2,4-decadienoate was attractive at 10-fold and 1,000-fold lower threshold dosages in the Petri-plate and in the Y-tube bioassays, respectively. Methyl (2E,4Z)-2,4-decadienoate was attractive to CM neonates in these bioassays at much higher doses than ethyl (2E,4Z)-2,4-decadienoate.“
[Attractants from Bartlett pear for codling moth, Cydia pomonella (L.), larvae., Knight, A.L., Light, D.M., Naturwissenschaften, 88(8), 2001, 339-342]
Masclef, A., Atlas des plantes de France, vol.2, t.111 (1890)
http://plantgenera.org/species.php?id_species=857515
