Dies ist eine alte Version des Dokuments!
Nicotiana tabacum L. - syn.Nicotiana chinensis Fisch. ex Lehm. - Solanaceae - tobacco, Virginischer Tabak
Annual herb, only known in cultivation, origin tropical South America.
The α-damascenone from tobacco extract and black tea is nearly optically inactive and present as a racemate with slight (R)-(+) excess.
[Chirospecific analysis in flavor and essential oil chemistry Part B. Direct enantiomer resolution of trans-α-ionone and trans-α-damascone by inclusion gas chromatography., Werkhoff, P., Bretschneider, W., Güntert, M., Hopp, R., Surburg, H., Zeitschrift für Lebensmittel-Untersuchung und Forschung, Vol.192(2), 1991, 111-115]
A large number of carotenoid degradation products are encountered in tobacco leaves, mainly the megastigmatrienones, damascenone, dihydractinodiolide, oxoeudalane and isophorone. Major acyclic isoprenoids are linalool, linalool oxide and geranyl acetone. The cembratriene-4,6-diols are the major cembranoids of tobaccos (Virginia tobacco: 0.23%), and the major degradation products of them are solanone (=virginione, (E)-5-isopropyl-8-methylnona-6,8-dien-2-one), solanol and norsolanadione. The green leaves contain (Z)-abienol, which during air- and sun-curing decreases significantly while numerous other labdanoids and labdanoid degradation products are formed, including the aroma intensive ambrox.
„… It is now known that norambreinolide [sclareolide] and related compounds, such as sclaral 3a,6,6,9a-tetramethyldodecahydronaphtho[2,1-b]furan-2-ol, are responsible for the characteristic cedar-amber notes of Havana leaf and Oriental tobacco.“
[Basic Chemical Constituents of Tobacco Leaf and Differences among Tobacco Types., Leffingwell, J.C., in: Tobacco: Production, Chemistry, And Technology, D.Layten Davis and Mark T.Nielson, Eds., Blackwell Science
(Pub.), 1999, Chapter 8A]
http://www.leffingwell.com/download/Leffingwell%20-%20Tobacco%20production%20chemistry%20and%20technology.pdf
Aroma properties of tobacco carotenoid derivatives reach from green-hay (safranal, β-cyclocitral), fruity (β-damascone and β-damascenone), sweet-floral (3-oxo-α-ionone), tea (theaspirone) to tobacco (oxo-edulan I and II, 4-oxo-β-ionone), and balsamic-violet-woody (α- and β-ionone).
[Tobacco., Leffingwell, J.C., Leffingwell Reports, Vol.2 (6), October, 2002]
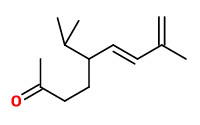 solanone | 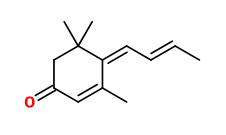 (6Z,8E)-megastigma-4,6,8-trien-3-one (tobacco cyclohexenone, tabanone - 4 isomers) |
The megastigma-4,6,8-trien-3-ones which elicit a tobacco-like, woody balsamic odor are considered key aroma components of Burley tobacco.
[Scent and Chemistry, Günther Ohloff, Wilhelm Pickenhagen, Philip Kraft, Wiley-VCH, 2012, 285-286]
(Burley tobacco condensate contains as much as 10% of 3,5,5-trimethyl-4-(2-butenylidene)-cyclohex-2-en-1-one)
[Novel Synthesis of 3,5,5‐Trimethyl‐4‐(2‐butenylidene)‐cyclohex‐2‐en‐1‐one, a Major Constituent of Burley Tobacco Flavour., Demole, E., Enggist, P., Helvetica Chimica Acta, 57(7), 1974, 2087-2091]
„The major volatile flavor compounds of flue-cured and burley tobacco were similar such as neophytadiene, solanone, megastigmatrienone isomers, β-damascenone and β-ionone. On the other hand, volatile flavor compounds such as norambreinolide, sclareolide were specifically identified in oriental tobacco.“
[Lee, Jang-Mi, et al. „Comparison of the volatile flavor compounds in different tobacco types by different extraction methods.“ Journal of The Korean Society of Tobacco Science (2010)]
„Nicotiana tabacum trichomes produce two types of diterpenoids – the polycyclic labdanoids and the macrocyclic cembranoids – that together may represent up to 10% of the leaf dry weight in some cultivars and under appropriate culture conditions (Wagner, 1991). The major labdanoids are Z-abienol and labdene-diol, whereas the major cembranoids are α- and β-cembratrien-diol (CBT-diols), together with generally smaller quantities of their respective precursors, α- and β-cembratrien-ol (CBT-ols) (Severson et al., 1984).“
[Characterization of two genes for the biosynthesis of the labdane diterpene Z‐abienol in tobacco (Nicotiana tabacum) glandular trichomes., Sallaud, C., Giacalone, C., Töpfer, R., Goepfert, S., Bakaher, N., Rösti, S., Tissier, A., The Plant Journal, Vol.72(1), 2012, 1-17]
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-313X.2012.05068.x/full
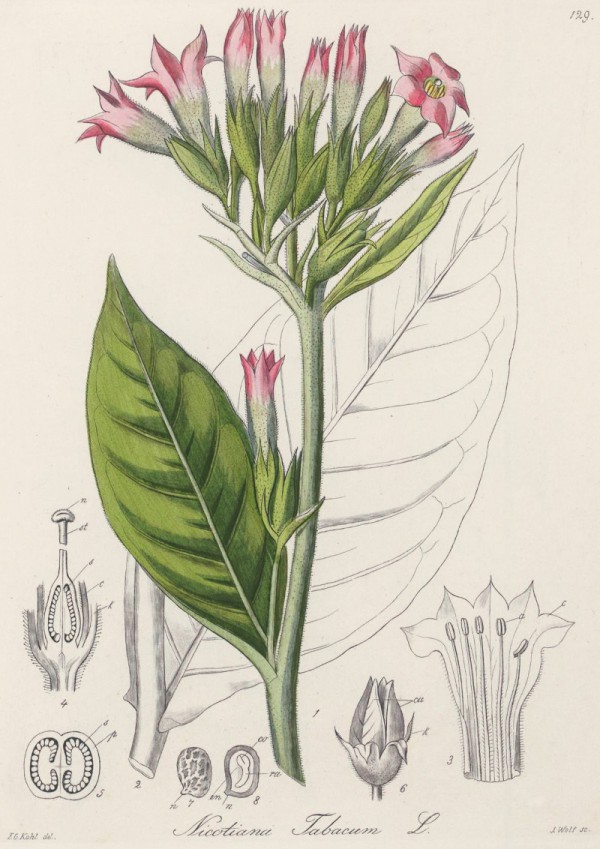
Kohl,F.G., Die officinellen Pflanzen der Pharmacopoea Germanica, t.129 (1891-1895) [F.G.Kohl]
http://plantgenera.org/species.php?id_species=700936
Nicotiana tabacum\ © Rolf Marschner (2010),
www.botanische-spaziergaenge.at
