Benutzer-Werkzeuge
Hyacinthus orientalis L. - syn.Hyacinthus praecox Jord. - Asparagaceae - (garden) hyacinth, (Garten-)Hyacinthe
Perennial herb, up to 20(40)cm high, native to Western Asia (Israel, Lebanon, Syria, Turkey), naturalized in the Mediterranean region, widely cultivated as ornamental; flowers in erect racemes, pendent to suberect, strongly sweetly scented, perianth white, pink or blue, 10-35mm, tube slightly constricted near middle, lobes strongly recurved.
http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?19427
Main constituents of the absolute of hyacinth flowers were benzyl alcohol (40.0%), (E)-cinnamyl alcohol (11.0%), benzyl acetate (8.1%), benzyl benzoate (6.0%), phenylethanol (3.7%), 1,2,4-trimethoxybenzene (3.0%), methyl eugenol (1.5%), 4-methoxyphenethyl alcohol (1.2%), and phenylethyl benzoate (1.2%). Other components included e.g. aldehydes like benzaldehyde (0.1%) and cinnamaldehyde (0.3%), alcohols like phenyl propyl alcohol (0.8%), aromatics like 1,4-dimethoxybenzene (0.2%) and 4-methoxyacetophenone (acetanisole; 0.1%), terpenes like α-farnesene (0.6%) and eugenol (0.2%), esters like benzyl salicylate (0.2%), methyl salicylate (0.1%) and cinnamyl acetate (0.7%), and olfactorly important minor and trace compounds like methyl N-methyl anthranilate (0.3%), elemicin (0.6%), indole (0.15%), 2-methoxy-3-isobutyl pyrazine (traces), and quinoline (traces).
[Kaiser, R. & Lamparsky, D., Nouveaux constituants de l'absolue de jacinthe et leur comportement olfactif. Parf. Cosm. Arômes, no. 17 (1977) 71-79]
Volatiles collected from the headspace of H.orientalis flowers included 2,6-dimethyl-3(E),5(Z),7-octatriene-2-ol and its 5(E) isomer (natural ratio 1:10, sweet floral odor) which are emitted at 8-12% of total volatiles in the evening and 1%-4% during day. (E)-ocimene as precursor showed the opposite rhythm (40-65% of total volatiles between hour 24-12).
[Rhythms of fragrance emission in flowers., Matile P., Altenburger R., Planta, 174, 1988, 242-247]
[Trapping, Investigation and Reconstitution of Flower Scents, Roman Kaiser, in: Müller, P.M., and Lamparsky, D. eds. Perfumes: Art, Science and Technology. Springer Science & Business Media, Dordrecht 1994, 213-250]
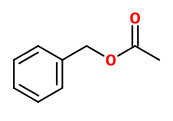 benzyl acetate (sweet floral) | 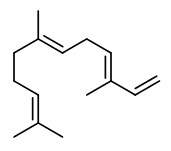 (E,E)-α-farnesene | 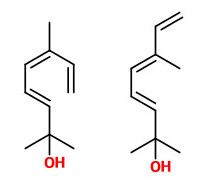 2,6-dimethyl-3(E),5(Z/E),7-octatriene-2-ol (sweet floral) | 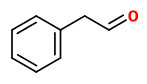 phenylacetaldehyde |
The principal constituents of the headspace (simultaneous close-loop stripping method, depending on absorbing agent) of unpicked white hyacinth flowers were benzyl acetate (16.3-31.2%), (E,E)-α-farnesene (16.2-31.7%), benzyl benzoate (5.1-14.5%), 2-phenylethanol (4.9-19.8%), (E)-ocimene (3.7-4.8%), and 1,2,4-trimethoxybenzene (2.8-6.7%). A large number of aromatic substances with influcence of the foral character of the hyacinth scent shown up, including e.g. p-methylanisole (0.1%), benzyl alcohol (0.5-3.0%), 2-phenylethyl acetate (1.5-2.3%), methyl benzoate (0.2-0.3%), and ethyl benzoate (0-0.1%). Compounds that may provide spicy aspects to the hyacinth fragrance were benzaldehyde (0.4-1.2%), (E)-cinnamyl alcohol (2.0-4.0%), phenyl propyl alcohol (dihydrocinnamyl alcohol, 0-0.4%), cinnamyl acetate (0.1-0.3%), eugenol (0-0.3%), and eugenol methyl ether (0.3-0.4%). Sweet animalic aspects are provided by N-methyl methyl anthranilate (0-0.05%), and indole (0-0.3%); while 1-octen-3-ol (0.9-1.7%) is associated with an earthy mushroom note. When Tenax is used rather than charcoal, phenylacetaldehyde (0.6-1.0%) with its strong green-floral odor (used for many years for the creation of synthetic hyacinth fragrances) was found at comparably high concentrations. On the other hand, the presence of considerable amounts of isomeric 2,6-dimethylocta-1,3,5,7-tetraenes and 2,6-dimethylocta-3,5,7-triene-2-ols was associated with charcoal as adsorbing material. It was shown that these compounds were artefacts from ocimene which mainly arise during sampling with one specific charcoal. GC-sniffing analysis showed that trace components are of great significance from the sensory point of view. These most potent compounds with extremely low odor tresholds were 2-isobutyl-3-methoxypyrazine (roasty fatty, galbanum), 2-sec-butyl-3-methoxypyrazine and 2-isobutyl-3-methoxypyrazine (green peas, galbanum).
[Headspace analysis of hyacinth flowers. Brunke, E. J., Hammerschmidt, F. J., Schmaus, G., Flavour and fragrance journal, Vol.9(2), 1994, 59-69]

Step,E., Bois,D., Favourite flowers of garden and greenhouse, vol.4 t.277 (1896-1897)
http://plantgenera.org/species.php?id_species=539956

hyacinth, CC BY-SA 3.0, Author: Andreas Kraska
Hyacinthus orientalis
© Rolf Marschner (2015),
www.botanische-spaziergaenge.at
