Dies ist eine alte Version des Dokuments!
Citrus paradisi - Rutaceae - grapefruit, Grapefrucht, Pampelmuse
Evergreen tree, usually 5-6(-15)m high; hybrid of Citrus × sinensis and Citrus maxima; widely cultivated in tropics and subtropics; leaves dark green, elliptic-ovate, midrib and veins glabrous, up to 15cm long; flowers fragrant; fruit 10-15 cm in diameter, peel greenish or yellow-orange; pulp mildly bitter.
Maybe created by spontaneous crossing of pamplemousse and orange in Barbados in the mid-18th century.
[Friedrich Zeller: Herkunft, Diversität und Züchtung der Banane und kultivierter Zitrusarten, Januar 2005.] http://www.uni-kassel.de/upress/online/frei/978-3-89958-116-4.volltext.frei.pdf
Naringin is the major flavonoid in grapefruit and gives the grapefruit juice its bitter taste. It is metabolized to the cytochrome P450 inhibiting flavanone naringenin in the human body. https://en.wikipedia.org/wiki/Naringin
„By application of the aroma extract dilution analysis on an extract prepared from fresh grapefruit juice, 37 odor-active compounds were detected in the flavor dilution (FD) factor range of 4−256 and subsequently identified. Among them the highest odor activities (FD factors) were determined for ethyl butanoate, 1-p-menthene-8-thiol, (Z)-3-hexenal, 4,5-epoxy-(E)-2-decenal, 4-mercapto-4-methylpentane-2-one, 1-heptene-3-one, and wine lactone. Besides the 5 last mentioned compounds, a total of 13 further odorants were identified for the first time as flavor constituents of grapefruit. The data confirmed results of the literature on the significant contribution of 1-p-menthene-8-thiol in grapefruit aroma but clearly showed that a certain number of further odorants are necessary to elicit the typical grapefruit flavor.“
[Characterization of the Most Odor-Active Volatiles in Fresh, Hand-Squeezed Juice of Grapefruit (Citrus paradisi Macfayden), Andrea Buettner and Peter Schieberle, J. Agric. Food Chem., 47 (12), 1999, 5189-5193]
Of thirty-eight aroma-active compounds detected in commercial cold-pressed grapefruit oil using GC-O and GC-MS (DB-Wax, DB-1), compounds with the greatest aroma intensities included 1,8-cineole, octanal, dodecanal, trans-4,5-epoxy-(E)-2-decenal, β-sinensal and nootkatone. „The aroma activities of sulphur compounds such as 4-mercapto-4-methyl-2-pentanol and methional were surprisingly low and neither of the two putative grapefruit aroma impact compounds, 4-mercapto-4-methyl-2-pentanone and 1-p-menthene-8-thiol, was detected.“
[Characterization of aroma‐impact compounds in cold‐pressed grapefruit oil using time–intensity GC-olfactometry and GC-MS., Lin, J., Rouseff, R.L., Flavour and Fragrance Journal, 16(6), 2001, 457-463]
„Twenty-five odor-active compounds were quantified in the fresh, hand-squeezed juice of White Marsh seedless grapefruits using stable isotope dilution assays. By calculation of the odor activity values of the odorants (ratio of their concentrations in the juice to their odor thresholds in water) it was shown that the fruity esters ethyl 2-methylpropanoate, ethyl butanoate, and (S)-ethyl 2-methylbutanoate, and the fruity, sweet winelactone, as well as the grassy smelling (Z)-hex-3-enal, and trans-4,5-epoxy-(E)-dec-2-enal with metallic odor, were among the most potent odorants of the fresh grapefruit juice. The typical sulfurous, grapefruit-like odor quality was mainly due to the catty, blackcurrant-like 4-mercapto-4-methylpentan-2-one and the grapefruit-like smelling 1-p-menthene-8-thiol. These findings were confirmed by reconstitution experiments to simulate the aroma of the fresh grapefruit juice.“
[Evaluation of key aroma compounds in hand-squeezed grapefruit juice (Citrus paradisi Macfayden) by quantitation and flavor reconstitution experiments., Buettner, A., Schieberle, P., Journal of Agricultural and Food Chemistry, 49(3), 2001, 1358-1363]
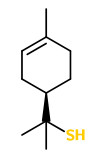
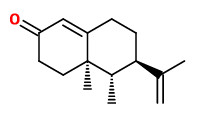
„The sesquiterpene ketone (+)-nootkatone, which possesses a citrus-like character and a bitter taste, also occurs as a typical constituent in almost all peel oils. [grapefruit 0.5-1.8%, grapefruit juice 3.3%] Due to its low odor treshold of 800μg/l water, this bicyclic sesquiterpene contributes much to the overall character of an essential oil even at concentrations below 0.001% (10.000ppb). Its enantiomer (-)-nootkatone lacks both the citrus character and the low treshold… The characteristic fresh fruity top-note of grapefruit juice is mainly due to p-menth-1-ene-8-thiol, which is present only in concentrations below 1ppb. [This] Compound has an odor treshold of 0.1ng/l water, … one of the lowest odor tresholds of all naturall aroma chemicals.“
[Scent and Chemistry, Günther Ohloff, Wilhelm Pickenhagen, Philip Kraft, Wiley-VCH, 2012, 222ff]
„The chemical composition of the essential oil (EO) obtained by solvent-free microwave extraction (SFME) and hydrodistillation (HD) from the peel of grapefruit (Citrus Paradisi. L) was analysed by gas chromatography/mass spectrometry (GC/MS). Totally, twenty-five components were identified in the EO. Limonene was observed as dominant (91.5-88.6%) for two extraction methods, SFME and HD, respectively. β-Pinene (0.8-1.2%), linalool (1.1-0.7%), α-terpinene (0.7-1.0%) and the other minor components were also detected. Disc diffusion method was applied to determine the antibacterial properties of the EO. The results showed that the EO of grapefruit peel had a wide spectrum of antimicrobial activities against Staphylococcus aureus, Enterococcus faecalis, Staphylococcus epidermidis, Escherichia coli, Salmonella typhimurium, Serratia marcescens and Proteus vulgaris, with their inhibition zones ranging from 11 to 53 mm.“
[Essential oil composition and antibacterial activity of the grapefruit (Citrus Paradisi. L) peel essential oils obtained by solvent‐free microwave extraction: comparison with hydrodistillation. Uysal, B., Sozmen, F., Aktas, O., Oksal, B. S., Kose, E. O., International Journal of Food Science & Technology, Vol.46(7), 2011, 1455-1461]

Citrus paradisi photo by Jeff Vanuga, USDA Natural Resources Conservation Service, PD (CC0)
https://commons.wikimedia.org/wiki/File:NRCSAZ02039_-_Arizona_(361)(NRCS_Photo_Gallery).tif