Benutzer-Werkzeuge
Boswellia carteri Birdw. - Burseraceae - frankincense, Afrikanischer Weihrauchbaum
Small tree, native to Somalia. The dried resin (Frankincense) is used in incense, perfumes, and medicinally.
Major components of the pale yellow essential oil of Boswellia carteri resin (hydrodistilled) were (+)-α-thujene (1.7%), (-)-α-pinene (10.9%), camphene (1.0%), sabinene (0.7%), β-pinene (0.7%), myrcene (0.5%), hexyl acetate (0.3%), p-cymene (1.4%), Z-β-ocimene (0.4%), E-β-ocimene (1.7%), (-)-limonene (1.5%), 1,8-cineole (1.2%), 1-octanol (11.9%), (-)-linalool (2.1%), α-pinene epoxide (0.5%), trans-verbenol (0.4%), terpinene-4-ol (0.4%), octyl acetate (39.3%), (-)-bornyl acetate (2.2%), geranyl acetate (0.4%), E-nerolidol (0.2%), cembrene A (2.1%), cembrene C (0.1%), verticilla-4(20),7,11-triene (6.0%), incensole (1.0%) and incensole acetate (2.3%).
[Basar, Simla. „Phytochemical investigations on Boswellia species.“ Comparative studies on the essential oils, pyrolysates and boswellic acids of Boswellia carterii, 2005, 42]
Main volatile components of B.carteri resin detected by headspace SPME-GC/MS were α-pinene (6-22%), limonene (10-22%), β-caryophyllene (6-16%), caryophyllene oxide (2-13%), β-myrcene (4%), and α-humulene (1-5%). Isoincensole and its acetate were present at 1.4-2.1%.
[A chemical investigation by headspace SPME and GC-MS of volatile and semi-volatile terpenes in various olibanum samples. Hamm, S., Bleton, J., Connan, J., & Tchapla, A., Phytochemistry, Vol.66(12), 2005, 1499-1514]
Main components identified in B.carteri oil were α-pinene (15.1%), myrcene (8.2%), limonene (18.2%) and α-cedrene (6.1%).
[Chemical Composition and Antimicrobial Activity of Some Oleogum Resin Essential Oils from Boswellia SPP. (Burseraceae), Lorenzo Camarda, Talya Dayton, Vita Di Stefano, Rosa Pitonzo, Domenico Schillaci, Annali di Chimica, Vol.97(9), 2007, 837–844]
„Major botanical and scientific references currently identify two species of frankincense, Boswellia carterii and Boswellia sacra, as being synonymous ( like NPGS; A.K.). We evaluated the Somalian (B. carterii) and Omani/Yemeni (B. sacra) species by chemical analyses to determine if there were any minor or major differences between the two species of frankincense. Components identified with their average percent for B. sacra are α-thujene (0.6%), α-pinene (68.2%), camphene (2.1%), sabinene (2.9%), β-pinene (2.0%), myrcene (0.7%), limonene + β-phellandrene (6.2%). Components identified with their average percent for B. carterii are α-thujene (7.9%), α-pinene (37.3%), camphene (0.8%), sabinene (4.9%), β-pinene (1.8%), myrcene (7.3%), limonene + β-phellandrene (14.4%). Initially, GC–MS analysis did not reveal major statistical differences. However, optical rotation values, B. Sacra (+30.1°) and B. carterii (−13.3°), demonstrated a greater significant difference. Enantiomeric ratio (+)/(−) values of α-pinene for B. sacra and B. carterii are 8.24 and 0.68, respectively, were also calculated aiding our conclusion that B. sacra and B. carterii are not synonymous but rather two distinct and individual frankincense species.“
[Chemical differentiation of Boswellia sacra and Boswellia carterii essential oils by gas chromatography and chiral gas chromatography-mass spectrometry, Cole L. Woolley et al., Journal of Chromatography A, Vol.1261, 26, 2012, 158-163]
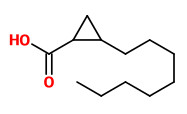
The (1S,2S)-(+)-trans- and (1S,2R)-(+)-cis-2-octylcyclopropyl-1-carboxylic acids (olibanic acids) were
detected in ppm amounts in all extracts obtained from B. carterii. These acids provide the very characteristic old churchlike endnote of the frankincense odor.
[Cerutti‐Delasalle, C., Mehiri, M., Cagliero, C., Rubiolo, P., Bicchi, C., Meierhenrich, U. J., & Baldovini, N. (2016). The (+)‐cis‐and (+)‐trans‐Olibanic Acids: Key Odorants of Frankincense. Angewandte Chemie, 128(44), 13923-13927]
„The immunomodulatory bioassay-guided fractionation of the oleogum resin of frankincense (Boswellia carterii
Birdwood) resulted in the isolation and identification of 9 compounds; palmitic acid and eight triterpenoids belonging to lupane, ursane, oleanane, and tirucallane skeleta were isolated form the resin. These triterpenoids are lupeol, β-boswellic acid, 11-keto-β-boswellic acid, acetyl-boswellic acid, acetyl 11-keto-β-boswellic acid, acetyl-α-boswellic acid, 3-oxo-tirucallic acid, and 3-hydroxy-tirucallic acid. The structures of the isolated compounds were deduced based on spectroscopic evidences. The lymphocyte transformation assay of the isolated compounds proved that the total extract retained more activity than that of any of the purified compounds.“
[Immunomodulatory Triterpenoids from the Oleogum Resin of Boswellia carterii Birdwood, Farid A.Badria*, Botros R. Mikhaeil,Galal T. Maatooq, Mohamed M. A. Amer, Z. Naturforsch. 58c,2003, 505-516]
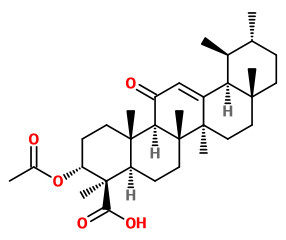 3-O-acetyl 11-keto-β-boswellic acid
3-O-acetyl 11-keto-β-boswellic acid
From the resin of B.carteri, two nuclear factor-κB (NF-κB) inhibitors were isolated, incensole acetate (IA) and its nonacetylated form, incensole (IN). „IA inhibited TAK/TAB-mediated IκB kinase (IKK) activation loop phosphorylation, resulting in the inhibition of cytokine and lipopolysaccharide-mediated NF-κB activation. It had no effect on IKK activity in vitro, and it did not suppress IκBα phosphorylation in costimulated T-cells, indicating that the kinase inhibition is neither direct nor does it affect all NF-κB activation pathways. The inhibitory effect seems specific; IA did not interfere with TNFα-induced activation of c-Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinase. IA treatment had a robust anti-inflammatory effect in a mouse inflamed paw model. Cembrenoid diterpenoids, specifically IA and its derivatives, may thus constitute a potential novel group of NF-κB inhibitors, originating from an ancient anti-inflammatory herbal remedy.“
[Incensole acetate, a novel anti-inflammatory compound isolated from Boswellia resin, inhibits nuclear factor-κB activation., Moussaieff, A., Shohami, E., Kashman, Y., Fride, E., Schmitz, M.L., Renner, F., Mechoulam, R., Molecular pharmacology, 72(6), 2007, 1657-1664] http://molpharm.aspetjournals.org/content/72/6/1657.full
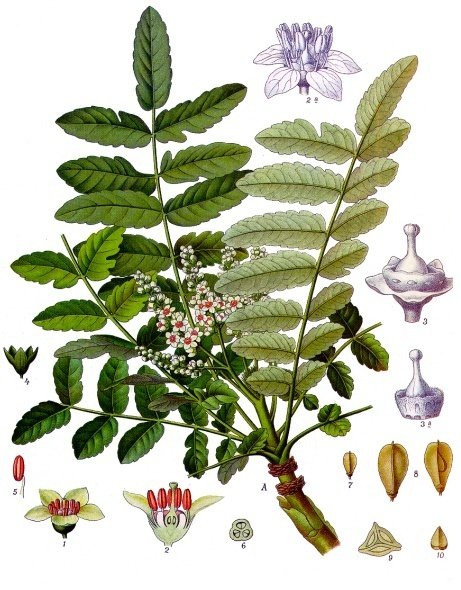
Boswellia carteri Birdw., Köhler, F.E., Medizinal Pflanzen, vol.2, t.175 (1890)