Dies ist eine alte Version des Dokuments!
Actinidia arguta (Siebold & Zucc.) Planch. ex Miq. - Actinidiaceae
hardy kiwi, kiwi berry, baby kiwi, Scharfzähniger Strahlengriffel, Japanische Stachelbeere, Kleinfrüchtige Kiwi
Perennial vine, native to Northern China, Korea and Japan.
„…edible, berry or grape-sized fruit similar to kiwifruit in taste and appearance, but are green, brownish, or purple with smooth skin, sometimes with a red blush. Often sweeter than the kiwifruit, hardy kiwifruit can be eaten whole and need not be peeled.“ https://en.wikipedia.org/wiki/Actinidia_arguta
„More than 240 compounds were detected when the volatile components of the flowers and the fruit from several Actinidia arguta genotypes were investigated. Around 60–70 different compounds were extracted from individual tissues of each genotype. Two different methods of volatile sampling (headspace and solvent) favoured different classes of compounds, dependent upon their volatilities and solubilities in the flower or fruit matrices. The compounds extracted from flowers largely comprised linalool derivatives including the lilac aldehydes and alcohols, 2,6-dimethyl-6-hydroxyocta-2,7-dienal, 8-hydroxylinalool, sesquiterpenes, and benzene compounds that are presumed metabolites of phenylalanine and tyrosine. Extracts of fruit samples contained some monoterpenes, but were dominated by esters such as ethyl butanoate, hexanoate, 2-methylbutanoate and 2-methylpropanoate, and by the aldehydes hexanal and (E)-2-hexenal. A number of unidentified compounds were also detected, including 8 from flowers that are so closely related that they are either isomers of one compound or two or more closely related compounds. This is the first report of the presence of a range of linalool derivatives in Actinidia.“
[Actinidia arguta: volatile compounds in fruit and flowers., Matich, A. J., Young, H., Allen, J. M., Wang, M. Y., Fielder, S., McNeilage, M. A., MacRae, E. A., Phytochemistry, Vol.63(3), 2003, 285-301]
„Biosynthesis of lilac compounds in ‘Hortgem Tahi’ kiwifruit (Actinidia arguta) flowers was investigated by treating inflorescences with d5-linalool. The incorporation of the deuterium label into 8-hydroxylinalool, 8-oxolinalool, the lilac aldehydes, alcohols, and alcohol epoxides was followed by GC-MS and enantioselective GC-MS. Both (R)- and (S)-linalool were produced naturally by the flowers, but 8-hydroxylinalool, 8-oxolinalool, and the lilac aldehydes and alcohols occurred predominantly as the (S) and 5′(S)-diastereoisomers, respectively. The enantioselective step in the biosynthesis of the lilac aldehydes and alcohols was concluded to be the oxidation of linalool to (S)-8-hydroxylinalool. In contrast, the lilac alcohol epoxides had a 5′(R):(S) ratio, the same as for linalool, which suggests that either these compounds are not synthesised from the 5′(S)-configured lilac aldehydes and alcohols, or that if indeed they are, then it is by an enantioselective step that favours utilisation of the 5′(R)-configured compounds.“
[Chirality and biosynthesis of lilac compounds in Actinidia arguta flowers., Matich, A. J., Bunn, B. J., Comeskey, D. J., Hunt, M. B., Rowan, D. D., Phytochemistry, Vol.68(13), 2007, 1746-1751]
„Kiwifruit (Actinidia spp. Lindl.) flowers and fruit contain many compounds of interest to the flavour and fragrance industries. In particular, Actinidia arguta (Sieb. et Zucc.) Planch. ex Miq. flowers produce β-linalool and important derivatives thereof, including linalool oxides, lilac aldehydes, alcohols and alcohol epoxides. Dynamic headspace sampling of whole A. arguta flowers showed that the peak emission rate of linalool, lilac alcohols and lilac aldehydes occurred around 0800 hours. After solvent extraction, linalool levels remained constant throughout the day and night, but lilac alcohol levels peaked at noon. In whole flowers, linalool was found predominantly in pistils and petals, and the lilac compounds were found mainly in petals. Two highly homologous (96.6% nucleotide identity) terpene synthase cDNA sequences, AaLS1 and ApLS1, were isolated from A. arguta and Actinidia polygama (Sieb. et Zucc.) Maxim flower EST libraries respectively. Real-time PCR analysis revealed that AaLS1 was expressed constitutively throughout the day and night, and primarily in petal tissue. Functional analysis in Escherichia coli showed that AaLS1 and ApLS1 each encoded a linalool synthase which was confirmed by transient expression in planta. Enantioselective gas chromatography revealed that both terpene synthases produced only (S)-(+)-linalool. AaLS1, therefore, is likely to be the key enzyme producing the (S)-linalool precursor of the lilac alcohols and aldehydes in A. arguta flowers.“
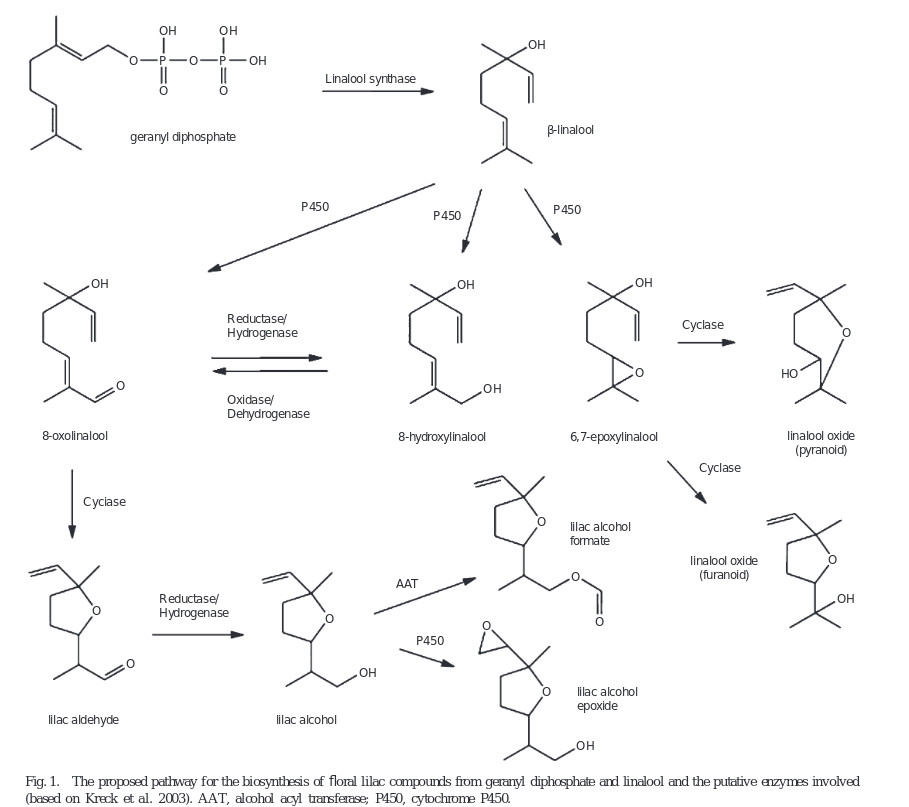
[Characterisation of an (S)-linalool synthase from kiwifruit (Actinidia arguta) that catalyses the first committed step in the production of floral lilac compounds., Chen, X., Yauk, Y.K., Nieuwenhuizen, N.J., Matich, A.J., Wang, M.Y., Perez, R.L., Beuning, L.L., Functional Plant Biology, Vol.37(3), 2010, 232-243]
Major compounds of the glycosidically bound volatile fraction of baby kiwi (Actinidia arguta) were 2,5-dimethyl-4-hydroxy-3(2H)-furanone (furaneol), benzyl alcohol, 3-hydroxy-β-damascone, hexanal, and (Z)-3-hexen-1-ol. „Eugenol, raspberry ketone, and 4-vinylguaiacol were identified for the first time in the fruit of an Actinidia species. The high concentration of 2,5-dimethyl-4-hydroxy-3(2H)-furanone in bound form (95.36 μg/kg) is particularly interesting and justifies further investigation.“
[Garcia, Coralia V., et al. „Characterization of the bound volatile extract from baby kiwi (Actinidia arguta).“ Journal of Agricultural and Food chemistry 59.15 (2011): 8358-8365]

Baby kiwi fruiting, CC BY-SA 3.0, Author: Andreas Kraska
