Syringa vulgaris L. - Oleaceae - common lilac, European lilac, Gemeiner Flieder
Deciduous shrub, 2-10m tall, native to Southeast Europe, cultivated since ancient times, now distributed all over the temperate zones; leaves entire, oval, subcordate, 5-10 cm long; flowers scented, corolla tube narrowly cylindric, lilac, white, azure, or red; fruit a smooth brown capsule. http://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=220013210
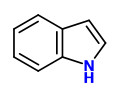
(E)-Ocimene and (Z)-ocimene were the most prominent compounds present in the headspace of lilac flowers, other main components were e.g. α-pinene, β-pinene, 1,4-dimethoxybenzene, and linalool. Minor components included camphene, limonene, camphor, β-bourbonene, 4-methylanisole, phenylacetaldehyde, estragole, 4-methyl acetophenone, 2-methoxybenzyl methyl ether, methyl eugenol, 1,2,4-trimethoxybenzene, piperonal, benzyl tiglate, (Z)-hexenyl benzoate, and indole.
[Lamparsky, D. „Headspace technique as a versatile complementary tool to increase knowledge about constituents of domestic or exotic flowers and fruits.“ Essential Oils and Aromatic Plants. Springer Netherlands, 1985, 79-92]
Main components of the headspace of living lilac flowers were 1,4-dimethoxybenzene (48.6%), (E)/(Z)-ocimene (11.0%), indole (2.8%), lilac aldehydes (2.1%), benzaldehyde (1.2%), nonanal (0.9%), benzyl methyl ether (0.8%), lilac alcohols (0.7%), 6-methyl-5-hepten-2-one (0.4%), rose furan (0.3%), and octanal (0.2%).
[Mookherjee BD et al., „Fruits and Flowers: Live vs Dead - Which do we want?“, in: Nishimura, O. „Flavors and Fragrances, a world perspective.“ Proceedings of the 10th international congress of essential oils, fragrances and flavors, Washington, DC. Vol. 375. 1986, 415-424]
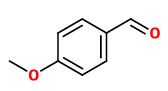
Major constituents of the vacuum headspace concentrate from the flowers were (E)-ocimene (38%), 1,4-dimethoxybenzene (9.7%), (Z)-3-hexenol (9.5%), lilac aldehydes (7.8%), lilac alcohols (4%), anisaldehyde (2.4%), and indole (1.8%). Minor amounts of unusual ethers were present: 1,2,4-trimethoxybenzene (0.5%), methylbenzylether (0.1%), 1,2-dimethoxybenzene (tr), and 2-methoxymethylanisole (tr).
[Joulain, D. „Study of the fragrance given off by certain springtime flowers.“ Progress in essential oil research (1986): 57-67]
The headspace of purple lilac flowers contained indole (2.3% living; 1.5% picked)
[Mookherjee, B. D., and Richard A. Wilson. „Tobacco constituents: Their importance in flavor and fragrance chemistry.“ Perfum. Flavor 15.1 (1990): 27-49]
The bark of S.vulgaris contains syringin, the „peculiarly disgusting, more sweet and scratching than bitter“ tasting glucoside of sinapyl alcohol (precursor to lignin or lignans).
[Ueber das Syringin., Bernays F.J., Pharmaceutisches Central Blatt, Vol.12, 1841, 938-939]
Syringin enhanced glucose utilization and lowered plasma glucose level in rats suffering artificially from insulin deficiency.
[Hypoglycemic Effect of Syringin from Eleutherococcus senticosus in Streptozotocin-Induced Diabetic Rats., Ho-Shan Niu, I-Min Liu, Juei-Tang Cheng, Che-Ling Lin, Feng-Lin Hsu, Planta Medica. Vol.74, 2008, 109-113]
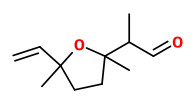
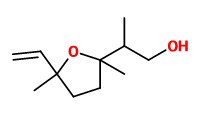
„The floral aroma of S. vulgaris variginata consists largely of aromatic aldehydes (benzaldehyde, phenylacetaldehyde, and p-anisaldehyde), an aromatic alcohol (2-phenylethyl alcohol), an aromatic ether (benzyl methyl ether), monoterpene aldehydes (lilac aldehyde and its isomer), monoterpene alcohols
(linalool, lilac alcohol and its isomer), an oxygenated monoterpene (1,8-cineole), monoterpene hydrocarbons (α-pinene and 3,7-dimethyl-1,3,6-octatriene), a sesquiterpene (α-farnesene), and a nitrogen-containing compound (indole). Eighteen compounds were identified, especially α-pinene, benzaldehyde, phenylacetaldehyde, linalool, 2-phenylethyl alcohol, lilac aldehyde isomers, lilac alcohol isomers, indole, and α-farnesene were found as the characteristic components of pale purple and white colors of S. vulgaris variginata. Lilac aldehydes [diastereomer of 2-(1-formyl)ethyl-5-methyl-5-vinyl tetrahydrofuran] and lilac alcohols [diastereomer of 2-(1-hydroxymethyl)ethyl-5-methyl-5-vinyl tetrahydrofuran] could be specified as the characteristic monoterpenes in S. vulgaris L. flowers with a desirable influence on the lilac fragrance quality. It was already reported that the metabolism of linalool and linalool derivatives can lead to the formation of lilac alcohols. In S. vulgaris only four out of all eight lilac aldehydes as well as alcohols were found [30]. Lilac aldehyde is of special interest for pollinators. It is emitted in high amounts, especially in nocturnal plant species, and it is known to be highly attractive to the nocturnal moth species. In pale purple and white colors of S. vulgaris variginata, lilac aldehydes (7.2% and 42.5%), lilac alcohols (17.8% and 13.5%) were found as characteristic components, respectively.“
[Rapid determination of floral aroma compounds of lilac blossom by fast gas chromatography combined with surface acoustic wave sensor., Se Yeon Oh, Hyun Du Shin, Sung Jean Kim, Jongki Hong, Journal of Chromatography A, Vol.1183, 2008, 170–178]
Lilac flowers emitted (ng/min) mainly (E)-β-ocimene (117), 1,4-dimethoxy benzene (80), and lilac aldehyde A (17), accompanied by benzaldehyde (13), benzyl methyl ether (13), (Z)-β-ocimene (11), lilac alcohols (9), acetophenone (4), (S)-(+)-linalool (5), lilac aldehyde B (3), estragole (3), 4-methylanisole (1), benzyl alcohol (2) and traces of phenylacetaldehyde.
[Floral to green: mating switches moth olfactory coding and preference. Saveer, Ahmed M., et al., Proceedings of the Royal Society B: Biological Sciences (2012): rspb20112710.]
http://rspb.royalsocietypublishing.org/content/early/2012/02/01/rspb.2011.2710.full.html
The major naturally occurring (5′S)-stereoisomers of lilac aldehydes (sweet, fresh, flowery, pleasant; ODT 0.2-0.4ng/L) have a lower odour threshold by 1-2 orders of magnitude in comparison to lilac aldehydes with (5′R)-absolute configuration (fresh, flowery; ODT 4-20ng/L).
[Dacho, Vladimír, and Peter Szolcsányi. „Synthesis and olfactory properties of seco-analogues of lilac aldehydes.“ Molecules 26.23 (2021): 7086.]

Syringa vulgaris L. as Lilac vulgaris (L.) Lam.
Duhamel du Monceau,H.L., Traité des arbres et arbustes, Nouvelle édition [Nouveau Duhamel], vol.2, t.61 (1804) [P.J.Redouté]
http://plantgenera.org/species.php?id_species=991858

Lilac CC BY-SA 3.0, Author: Andreas Kraska