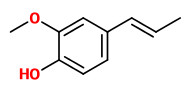
Quercus petraea (Matt.) Liebl. - syn. Quercus sessilis Ehrh.; Quercus sessiliflora Salisb. - Fagaceae
sessile oak, Irish oak, Welsh oak, Cornish oak, Durmast oak, Traubeneiche, Wintereiche, Steineiche
Deciduous monoecious tree, up to 35m high, native to Europe, West Asia; leaves 7-14cm long, petioled 1-3cm, evenly lobed; male inflorescence pendulous, up to 6cm long; female inflorescences in leaf axils, flowers in stalkless clusters of 2-5; fruit an acorn 2-3cm long.
„Seedlings of Q.petraea may be more shade-tolerant than those of Q.robur and is therefore more able to regenerate in woodland.“
[Quercus petraea (Sessile Oak), Online Atlas of the British and Irish flora] http://www.brc.ac.uk/plantatlas/index.php?q=plant/unmatched-species-name-250
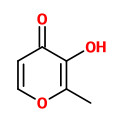
„Toasting wood to be used in barrels for aging wine and brandy produces a great number of volatile and odiferous compounds. Some of these have a “toasty caramel” aroma. Analysis by high-performance gas chromatography of toasted oak extracts, combined with olfactory detection, enabled various chromatographic peaks with these specific aromas to be isolated. These same odors were simultaneously studied by heating glucose both with and without proline. Aromatic compounds of interest were identified thanks to a combination of gas chromatography and both mass and infrared spectrometry. In addition to already-known substances such as 2-hydroxy-3-methyl-2-cyclopenten-1-one (cyclotene) and 3-hydroxy-2-methyl-4H-pyran-4-one (maltol), we identified, for the first time, 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, 4-hydroxy-2,5-dimethyl-3(2H)-furan-3-one (furaneol), and 2,3-dihydro-5-hydroxy-6-methyl-4H-pyran-4-one (dihydromaltol) as compounds which actively contribute to the “toasty caramel” aroma of heated oak.“
[Cutzach, Isabelle, et al. „Identification of volatile compounds with a “toasty” aroma in heated oak used in barrelmaking.“ Journal of Agricultural and Food Chemistry 45.6 (1997): 2217-2224]
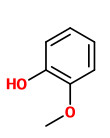
„Gas chromatography–mass spectrometry (GC-MS) and gas chromatography-olfactometry (GC-O) were used to study aroma‐active compounds in extracts of American, French, Hungarian and Russian oak woods. Compounds that presented high odour intensities for the non‐toasted oak woods were guaiacol, hexanal, trans‐2‐nonenal, trans‐oak lactone, cis‐oak lactone, eugenol, vanillin and trans‐isoeugenol, whilst the same compounds, in addition to furfural, 4‐methylguaiacol and cis‐isoeugenol, proved important in the toasted oaks. Like the oak lactones, cis‐ and trans‐isoeugenol presented woody/oak odours, particularly in the toasted samples. For Hungarian and Russian samples, both characterized by their lower content of oak lactones, trans‐ and cis‐isoeugenol presented higher odour intensities. For this reason, samples of low oak lactone concentrations, such as Hungarian and Russian oak woods, but also containing isoeugenols, can impart woody/oak odours to wines.“
[Díaz‐Maroto, M. Consuelo, et al. „Aroma‐active compounds of American, French, Hungarian and Russian oak woods, studied by GC-MS and GC-O.“ Flavour and fragrance journal 23.2 (2008): 93-98] https://onlinelibrary.wiley.com/doi/pdf/10.1002/ffj.1859
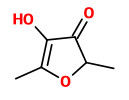
„A SPME-GC-MS method was adapted and validated in order to quantify 5 megastigmatrienones and related odorous compounds from oak wood: guaiacol, cis-whisky lactone, trans-whisky lactone, γ-nonalactone, eugenol, vanillin, and acetovanillone in a single run. The five megastigmatrienone isomers (tabanones) were quantified, for the first time, in Cognac, Armagnac and rum, as contributors to tobacco-like aromas. Spirits aged in oak barrels contain higher amounts, but megastigmatrienones are also present in freshly-distilled spirits. Statistical analysis revealed that freshly-distilled and barrel-aged spirits were differentiated by their megastigma-4,7E,9-trien-3-one levels. The Armagnac and Cognac samples were distinguished by their concentrations of the megastigma-4,6Z,8E-trien-3-one isomer.“
[Slaghenaufi, Davide, et al. „Quantification of megastigmatrienone, a potential contributor to tobacco aroma in spirits.“ Food Chemistry 203 (2016): 41-48]
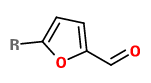
„Crude organic extracts from oak wood samples (toasted at different temperature–time couples) were analyzed by a sensory-guided approach using GC-O-TOFMS, followed by purification with semipreparative HPLC (reverse phase). This approach revealed two specific odorous zones (OZs) reminiscent of metal and puff pastry. The first OZ was identified as trans-4,5-epoxy-(E)-2-decenal (1) by coinjection of the commercial product, whereas identification of (2E,4E,6Z)-nonatrienal (2) associated with puff pastry OZ was validated by a multistep chemical synthesis approach (Wittig reaction) followed by semipreparative HPLC purification (chiral phase). Their detection thresholds in model wine solution were 60 ng/L (1) and 16 ng/L (2). Their distribution in toasted oak wood samples [GC-NCI-MS (NH3) analysis] ranged from some ng/g to 210 ng/g for (1) and 85 ng/g for (2). Finally, additional sensory experiments demonstrated the impact of newly identified aldehydes in toasted oak wood.“
[Courregelongue, Marie, et al. „Identification and distribution of new impact aldehydes in toasted oak wood (Quercus petraea).“ Journal of Agricultural and Food Chemistry 70.37 (2022): 11667-11677]
The proantho-cyanidins isolated from oak bark (Quercus petraea) „…had an average polymerization degree of 6.1 and a procyanidin: prodelphinidin ratio of 6:4. The prevailing units of the oligomeric chains were (+)-catechin, (-)-epicatechin, and (+)-gallocatechin, minor units were (-)-epicatechin 3-O-gallate and (-)-epigallocatechin 3-O-gallate. The bark contains both hydrolyzable and condensed tannins. Although only 23% of the water-soluble oak bark tannins consisted of oligomeric proanthocyanidins, these contributed with 55% to the astringency of the total tannin fraction.“
[Proanthocyanidins from Quercus petraea Bark., Pallenbach, E., Scholz, E., König, M., Rimpler, H., Planta medica, 59(3), 1993, 264-268]
The bark contained th ellagitannins 2,3-(S)-hexahydroxydiphenoyl-glucose, pedunculagin, vescalagin, and castalagin; the flavanoellagitannins acutissimin A, acutissimin B, eugenigrandin A, guajavin B, and stenophyllanin C; and the procyanidinoellagitannin.
[Ellagitannins and complex tannins from Quercus petraea bark., König, M., Scholz, E., Hartmann, R., Lehmann, W., Rimpler, H., Journal of natural products, 57(10), 1994, 1411-1415]
The dried bark of young trees of Q.petraea and Q.robur (oak bark, Quercus cortex, Cortex Quercus, Eichenrinde) is used as decocotion internally against diarrhoea, externally against inflammations of the skin or mucosa, particularly of the genital and anal region (as sitting bath).
[Hagers Handbuch der Pharmazeutischen Praxis, Springer 2010]
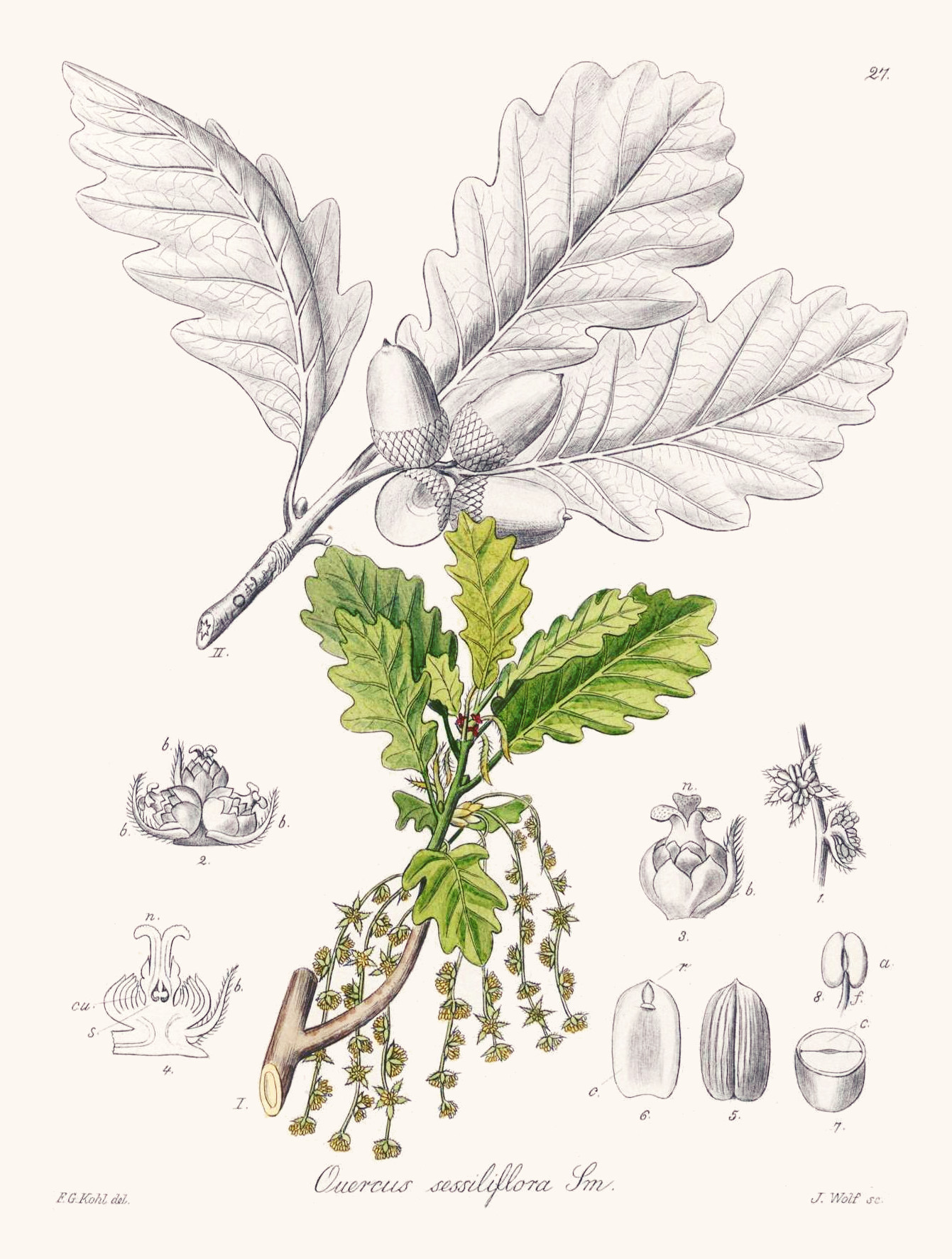
Quercus petraea (Matt.) Liebl. as Quercus sessiliflora Salisb.
Kohl,F.G., Die officinellen Pflanzen der Pharmacopoea Germanica, t.27 (1891-1895)
http://plantgenera.org/species.php?id_species=861250
Quercus petraea
© Rolf Marschner (2006),
www.botanische-spaziergaenge.at