Pastinaca sativa L.- syn.Pastinaca pratensis H.Mart.; Pastinaca opaca Bernh. - Apiaceae
parsnip, (Gemeiner) Pastinak, Hammelmöhre
Biennial herb, when cultivated usually grown as annual, up to 1m tall, native to Europe and Asia; taproot long, thick, white, edible; branched stem hollow, grooved, 30-120 cm tall; leaves alternate, made up of 5 -15 egg shaped leaflets along both sides of a common stalk; leaflets sharply-toothed or lobed at the margins; flowers yellow, in a loose, compound umbel; fruits oval, flat, straw colored.
Several varieties of P. sativa have been described, as:
Pastinaca sativa subsp. sativa var. pratensis (Pastinaca pratensis (Pers.)H.Martius) - wild parsnip, Wiesen-Pastinak
Pastinaca sativa subsp. sativa var. sativa - garden parsnip, Gemüse-Pastinak
Pastinaca sativa subsp. sylvestris (Pastinaca sylvestris Mill.)- wild parsnip, Zottiger Pastinak
http://de.wikipedia.org/wiki/Pastinak
http://www.botanik-bochum.de/html/jahrbuch/2012/Pflanzenportraet_Pastinaca_sativa.pdf
„The parsnip is usually cooked but can also be eaten raw. It is high in vitamins and minerals, especially potassium. It also contains antioxidants and both soluble and insoluble dietary fiber. It can be cultivated in deep, stone-free soils and is attacked by the carrot fly and other insect pests, viruses and fungal diseases, of which canker is the most serious. In sunlight, handling the stems and foliage can cause a skin rash… While the root of the parsnip is edible, handling the shoots and leaves of the plant requires caution as the sap is toxic. Like many other members of the family Apiaceae, the parsnip contains furanocoumarin, a photosensitive chemical that causes a condition known as phytophotodermatitis. The condition is a type of chemical burn rather than an allergic reaction, and is similar to the rash caused by poison ivy.“
http://en.wikipedia.org/wiki/Parsnip
http://www.dnr.state.mn.us/invasives/terrestrialplants/herbaceous/wildparsnip.html
By headspace examination of the salt-saturated juice from raw roots, 2-sec-butyl-3-methoxypyrazine (green earthy vegetable-like) has been found an olfactory important volatile component of parsnip.
[Murray, Keith E., and Frank B. Whitfield. „The occurrence of 3‐alkyl‐2‐methoxypyrazines in raw vegetables.“ Journal of the Science of Food and Agriculture 26.7 (1975): 973-986]
Myristicin is a main constituent (up to 44%) of the essential oil of P.sativa ssp.sativa var.hortensis roots.
[Variation of myristicin content in cultivated parsnip roots (Pastinaca sativa subspecies sativa var hortensis). Stahl, E. , Journal of agricultural and food chemistry, Vol.29(4), 1981, 890-892]
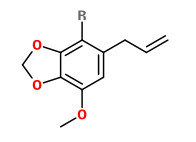 myristicin (R=H) | 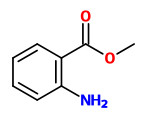 methyl anthranilate | 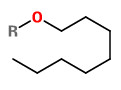 octanol (R=H) octyl acetate (R=Ac) |
Main volatile compounds of the floral fragrance of P.sativa, collected at PorapakQ, were E/Z-β-ocimene (61%) and methylanthranilate (12%). Interesting minor components were octyl butyrate, linalool and geranyl acetate eg.
[Volatile compounds from flowers of six species in the family Apiaceae: bouquets for different pollinators? Borg-Karlson, A.K., Valterová, I., Nilsson, L.A., Phytochemistry, Vol.35(1), 1993, 111-119]
„The aliphatic esters octyl acetate and octyl butyrate occur as major components of essential oils in the vittae, or oil tubes, of the wild parsnip (Pastinaca sativa). We determined phenotypic variation and narrow-
sense heritabilities of these octyl esters in wild parsnip fruits from 30 maternal families. The mean octyl acetate content was 1.56 μg/mg dry fruit (0.08-5.51 μg/mg dry fruit) and the mean octyl butyrate content
was 4.28 μg/mg dry fruit (1.28-14.22 μg/mg dry fruit)… mean content of furanocoumarines in mature wild parsnip fruits are bergapten 1.85 μg/mg, imperatorin 3.72 μg/mg, sphondin 0.31 μg/mg, xanthotoxin 4.02 μg/mg …“
[Heritability estimates for octyl acetate and octyl butyrate in the mature fruit of the wild parsnip. Carroll, M. J., A. R. Zangerl, and M. R. Berenbaum. Brief communication. Journal of Heredity Vol.91 (1), 2000, 68-71] http://jhered.oxfordjournals.org/content/91/1/68.full.pdf
„The floral bouquet of US parsnips comprised 16 distinct compounds, 14 of which could be identified; two
monoterpenes could not be definitively characterized. The most abundant constituents were cis- (30. 81%) and trans-trans ocimenes (23.69%). As in the US, the floral bouquet of P. sativa in NZ was dominated by cis-cis (32.27%) and trans- (49.12%) ocimenes and octyl butyrate. NZ umbels emitted low quantities (<1%) of five compounds absent in the US floral bouquet: linalool, carene, allo-allo ocimene and two isomers of α-farnesene. We found a total of 20 identifiable constituents but we were unable to identify two compounds present in low
concentrations. A comparison of US and NZ floral volatiles revealed significant differences in the proportional
representation tion of seven compounds; NZ flowers emit a greater proportion of beta-pinene, trans-ocimene,
ocimene, gamma-terpinene, gamma octyl butyrate and germacrene whereas US flowers emit a greater proportion of octanol and octyl acetate.“
[Implications of enemy escape on chemically mediated interactions with mutualists: wild parsnip pollination in two hemispheres. Tania Jogesh, Arthur Zangerl, Margaret C. Stanley, May R. Berenbaum, Journal of Pollination Ecology, 11(8), 2013, pp 57-67]
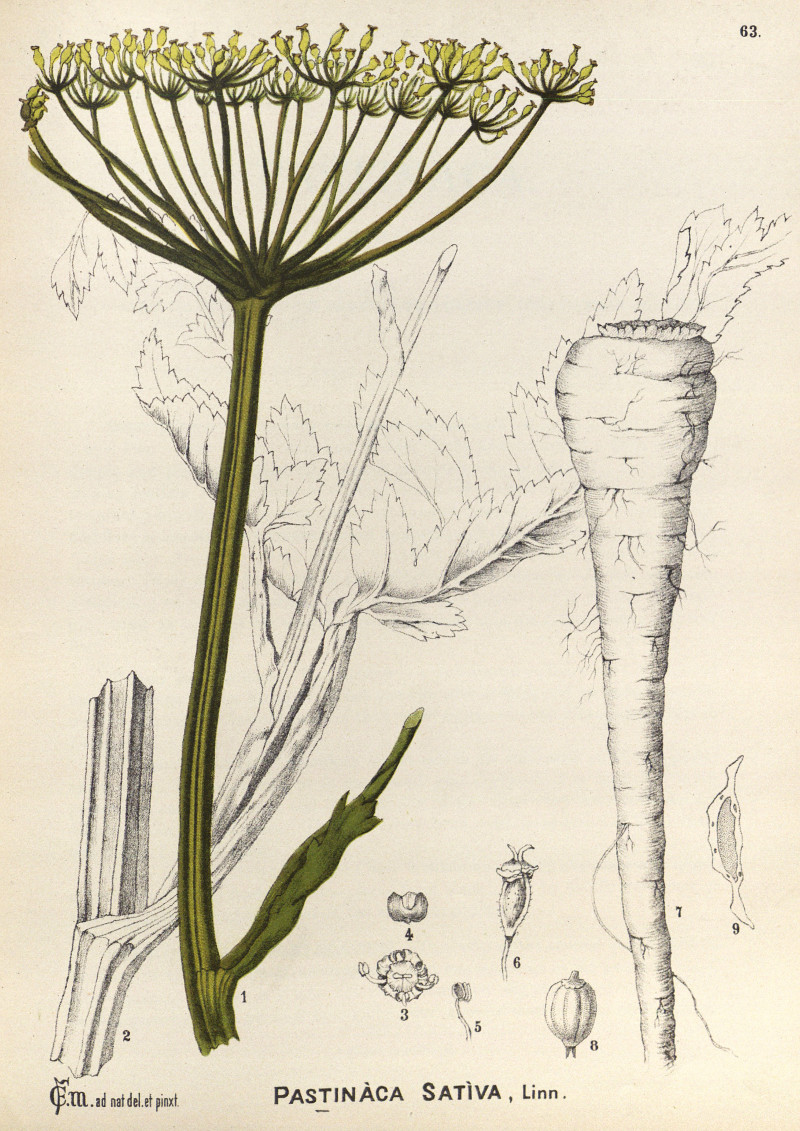
Millspaugh, C.F., American medicinal plants, vol.1, t.63 (1892)
http://plantgenera.org/species.php?id_species=755114