Passiflora edulis Sims - Passifloraceae - passionfruit, Maracuja, Passionsfrucht
Passiflora edulis Sims forma flavicarpa - yellow passionfruit, golden passionfruit, Maracuja
Passiflora edulis Sims forma edulis - purple passionfruit, purple granadilla, Purpurgrenadille, Maracuja
„… vine species of passion flower that is native to Brazil, Paraguay and northern Argentina… cultivated commercially in tropical and subtropical areas for its sweet, seedy fruit… The passion fruit is round to oval, either yellow or dark purple at maturity, with a soft to firm, juicy interior filled with numerous seeds. The fruit is both eaten and juiced; passion fruit juice is often added to other fruit juices to enhance aroma.“ http://en.wikipedia.org/wiki/Passiflora_edulis
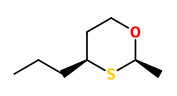
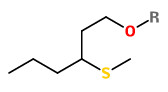
3-Methylthio-hexanol as well as (Z) and (E)-2-methyl-4-propyl-1,3-oxathiane were identified as character impact compounds in a flavor concentrate of the yellow passion fruit of Hawaiian origin.
[Identification and synthesis of two new organic sulfur compounds from the yellow passion fruit (Passiflora edulis f. flavicarpa)., Winter, M., Furrer, A., Willhalm, B., Thommen, W., Helvetica Chimica Acta, 59(5), 1976, 1613-1620]
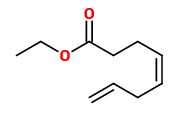
The strikingly fresh-fruity ethyl (Z)‐4,7‐octadienoate (pineapple-like) and (Z)‐3,5‐hexadienyl butyrate (tropical fruity) are important aroma constituents of the purple passionfruit.
[Winter, Max, et al. „(Z)‐4,7‐Octadiensäure‐äthylester und (Z)‐Buttersäure‐3,5‐hexadienylester, zwei neue Aromastoffe der roten Passionsfrucht.“ Helvetica Chimica Acta 62.1 (1979): 135-139]
The headspace of the juice from the hybrid passion fruit (purple skinned, smaller than the yellow one) showed by far higher ester content then the yellow and the red one. Main components of the headspace were esters (more than 30 different found) like ethyl butanoate, ethyl hexanoate, hexyl butanoate, and hexyl hexanoate. Minor components were e.g. hexanol, benzaldehyde, ethyl 4,7‐octadienoate, ocimene, edulan I, and β-ionone.
[Chen, Chu Chin, et al. „Headspace components of passion fruit juice.“ Journal of Agricultural and Food Chemistry 30.6 (1982): 1211-1215]
Enantioselective synthesis starting form (E)-2-hexenol showed that (+)- and (-)-(Z)-2-methyl-4-propyl-1,3-oxathiane exhibit different organoleptic properties. Whereas the (+)-enantiomer had a estery-camphoraceous-flowery type odor, (-)-(Z)-2-methyl-4-propyl-1,3-oxathiane smelled powerful sulfury, tropical-fruit-like (th 4ppm).
[Enantioselective synthesis of (+)‐and (-)‐cis‐2‐methyl‐4‐propyl‐1,3‐oxathiane and their olfactive properties., Pickenhagen, W., Brönner‐Schindler, H., Helvetica chimica acta, 67(4), 1984, 947-952]
The purple-skinned fruits of passiflora edulis and the yellow-skinned Passiflora edulis var.flavicarpa fruits „… are distinguished by a unique flavour; the purple passionfruit has an intensely pleasant, floral, fruity aroma, whereas the yellow variety has an exotic ester aroma with a sharp sulfury note.“
Sniffed by GC-O and calculated from its flavour impact value and its concentration in the juice, the most important flavour constituents of purple passionfruit juice were the megastigma-4,6,8-trienes, (Z)-3-hexenyl hexanoate, hexyl butanoate, ethyl (Z)-4-octenoate, β-ionone, edulan I, ethyl (Z)-4,7-octadienoate, linalool, sulfur compounds, rose oxide, (Z)-3-hexenol, hexanol/nonan-2-one, heptanol, ethyl hexanoate, and methyl butanoate.
[Whitfield, Frank B., and John H. Last. „The flavour of the passion fruit-a review.“ Progress in Essential Oil Research. Berlim: Gruyter (1986): 3-48]
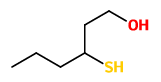
„Capillary gas chromatographic analysis of aroma extracts obtained by simultaneous distillation-extraction from yellow passion fruits (Passiflora edulis f. flavicarpa) using a flame photometric detector revealed the presence of previously unreported sulfur-containing volatiles. 3-Mercaptohexanol and the acetates, butanoates, and hexanoates of 3-mercaptohexanol and 3-(methylthio)hexanol, respectively, were identified by comparison of chromatographic and mass spectral data with those of synthesized reference compounds. Approximate amounts of these trace constituents in freshly harvested fruits and in commercial passion fruit products were determined. The newly identified compounds, especially the acetates, exhibit powerful sensory properties.“
[Identification of new sulfur-containing volatiles in yellow passion fruits (Passiflora edulis f. flavicarpa)., Engel, K.H., Tressl, R., Journal of agricultural and food chemistry (USA), Vol.39(12), 1991, 2249-2252]
„Sulfur-containing molecules are organoleptically by far the most interesting components in yellow passion
fruits. Recent work by Engel and Tressl (1991) revealed the sensory importance of 3-mercaptohexanol and 3-
(methylthio)hexanol as well as the acetates, butanoates, and hexanoates of these sulfur-substituted alcohols for the aroma of yellow passion fruits. Due to their high odor values and their olfactive profiles, these sulfur substances are expected to be key ingredients of the yellow variety… They are especially distinguished by their high intensity and long-lasting character. In addition to 3-mercaptohexyl- and 3-(methylthio)hexyl acetates, butanoates, and hexanoates, we were able to detect 3-mercaptohexyl pentanoate in
yellow passion fruit flavor… In conclusion, the flavor of yellow passion fruits is determined by numerous volatile components belonging to different classes of organic compounds. According to our investigations, however, the attractive tropical flavor note of ripe yellow passion fruits is mainly attributed to the presence of trace levels of sulfur volatiles in combination with other compounds contributing fruity, estery, floral, and green aroma impressions.“
[Vacuum headspace method in aroma research: flavor chemistry of yellow passion fruits., Werkhoff, P., Güntert, M., Krammer, G., Sommer, H., Kaulen, J., Journal of Agricultural and Food Chemistry, Vol.46(3), 1998, 1076-1093]
„Chemical characterization by gas chromatography-mass spectometry (GC-MS) of the aromatic profile of yellow passion fruit essence and the juice of the fruit yielded a total of 62 and 34 components, respectively… Aroma extract dilution analysis (AEDA) allowed for the detection of the most potent odorants in the commercial essence (2-methylbutyl hexanoate and hexyl hexanoate) and in the fresh juice (1,3-dimethyl benzene and 2-methylbutyl hexanoate). 2-Methylbutyl hexanoate, considered as one of the most potent odorants in this fruit, has been described for the first time as an aromatic constituent of yellow passion fruit.“
[Characterization of the aromatic profile in aqueous essence and fruit juice of yellow passion fruit (Passiflora edulis Sims f. flavicarpa Degner) by GC-MS and GC/O., Jordán, M.J., Goodner, K.L., Shaw, P.E., Journal of agricultural and food chemistry, Vol.50(6), 2002, 1523-1528]
The headspace of five fruit cultivars of yellow passion fruit contained enantiomerically pure (R)-(+)-γ-decalactone, but γ-nonalactone and γ-undecalactone were absent. Of the commerical „passion fruit“ products analysed, only one contained (R)-(+)-γ-decalactone as a dominant enantiomer (96%). Many commercial products contained racemic γ-decalactone and some even contain racemic mixtures of γ-undecalactone or γ-nonalactone. In two products γ-decalactone was absent.
[Ravid, Uzi, et al. „Authenticity assessment of natural fruit flavour compounds in foods and beverages by auto‐HS-SPME stereoselective GC-MS.“ Flavour and fragrance journal 25.1 (2010): 20-27]
„The volatile compositions from organic and conventional passion fruit pulps produced in Brazil were investigated. … The volatile compounds from the headspace of the passion fruit … were identified through gas chromatography/mass spectrometry… A total of 77 compounds were detected in the headspace of the passion fruit pulps - 60 of which were identified, comprising 91% of the total chromatogram area. The major compounds were the following: ethyl butanoate, 52% and 57% of the total relative area of the chromatogram for the organic and conventional passion fruit pulps; ethyl hexanoate, 22% and 9%; and hexyl butanoate, 2% and 5%. The aroma of the organic passion fruit pulp is mainly related to the following volatile compounds: ethyl hexanoate, methyl hexanoate, β-myrcene and D-limonene. The conventional passion fruit pulp presented methyl butanoate, butyl acetate, hexanal, 1-butanol, butyl butanoate, trans-3-hexenyl acetate, cis-3-hexen-1-ol, butyl hexanoate, hexyl butanoate, 3-hexenyl butanoate and 3-hexenyl hexanoate as the main volatile compounds for aroma.“
[Volatile compounds from organic and conventional passion fruit (Passiflora edulis F. Flavicarpa) pulp., Macoris, M.S., Janzantti, N.S., Garruti, Deborah dos Santos, Monteiro, M., Food Science and Technology (Campinas), Vol.31(2), 2011, 430-435]
http://www.scielo.br/scielo.php?pid=S0101-20612011000200023&script=sci_arttext
The floral scent of P.edulis is dominated by 1,4-dimethoxybenzene (44.7%). Other major compounds found in the headspace are benzenoids like benzyl alcohol (1.4%), 4-ethyl-benzaldehyde (1.7%), 3,4-dimethylacetophenone (1.1%), trans-methyl cinnamate (5.9%), and esters like butyl acetate (5.4%), allyl acetate (3.0%), and isobutyl acetate (2.4%). „Olfactive description: white flower, aspects of Ylang-Ylang, balsamic, anisic, carnation (eugenol, heliotropin), animalic (creolic, methyl benzoate notes) with citric freshness of pamplemouse.“
[Montero, D.A., Marques, M.O.M., Meletti, L.M., Kampen, M.H., Polozzi, S.C. (2016). Floral scent of brazilian Passiflora: five species analised by dynamic headspace. Anais da Academia Brasileira de Ciências, (AHEAD), 0-0.]

Passiflora edulis forma flavicarpa (left), forma edulis (right)
CC BY-SA 3.0, Author: Fibonacci Wikimedia Commons