Hyoscyamus niger L. - Solanaceae - common henbane, black henbane, stinking nightshade, Schwarzes Bilsenkraut
Biennial herb, up to 1m tall, native to Europe, West Asia, North Africa; rosette leaves ovate-lanceolate or oblong, coarsely dentate or pinnately lobed or parted, cauline leaves ovate or deltate-ovate, nearly clasping or broadly cuneate at base, lobed or entire; flowers pale yellow, with purple veins, campanulate, 2-3 cm; fruiting calyx urceolate; capsules ovoid-rounded; seeds yellow-brown, discoid, ca. 1 mm in diam.
Alkaloids (hyoscyamine and scopolamine) contained in the roots, leaves, and seeds, are used as an anaesthetic and for relieving muscular spasm and pain. The seed oil can be used for soap making.
http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=200020521
„Henbane leaf and the isolated hyoscamine (and atropine, its racemate) are used in modern phytotherapy mainly to treat spasms of the gastrointestinal tract. It is sometimes included as a sedative in herbal mixtures and combination products. The leaves were formerly smoked to treat asthma (hyoscyamine has bronchodilatory effects), in the same way as Atropa leaf or Datura leaf… The active compounds are the tropane alakaloids hyoscyamine (the major compound) and scopolamine (also in high yiled). Alkaloids occur in concentrations of 0.04-0.015% of dry weight in the leaves and up to 0.3% in the seeds. Cases of acccidental or deliberate poisoning are rare.“
[Medicinal Plants of the World. Ben-Erik Van Wyk and Michael Wink, Pretoria 2004, 174]
„Tropane alkaloids are valuable pharmaceutical drugs derived from solanaceous plants such as Hyoscyamus niger (black henbane). The biosynthesis of these molecules, including the nature of the enigmatic rearrangement of (R)-littorine to (S)-hyoscyamine, is not completely understood. To test the hypothesis that a cytochrome P450 enzyme is involved in this rearrangement, we used virus-induced gene silencing to silence a cytochrome P450, CYP80F1, identified from H. niger roots by EST sequencing. Silencing CYP80F1 resulted in reduced hyoscyamine levels and the accumulation of littorine. Hyoscyamine was observed in CYP80F1-expressing tobacco hairy roots supplied with (R)-littorine. Expression in yeast confirmed that CYP80F1 catalyzes the oxidation of (R)-littorine with rearrangement to form hyoscyamine aldehyde, a putative precursor to hyoscyamine, and without rearrangement to form 3′-hydroxylittorine. Our data strongly support the involvement of CYP80F1 in the rearrangement of littorine to hyoscyamine.“
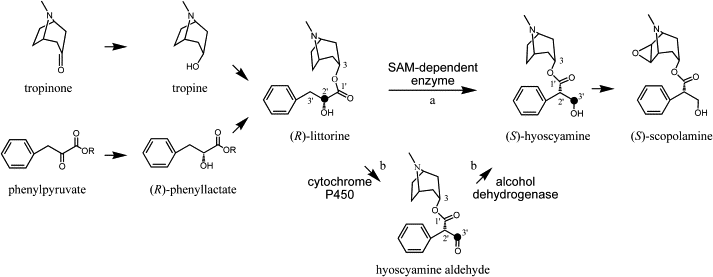
[Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement., Li, R., Reed, D.W., Liu, E., Nowak, J., Pelcher, L.E., Page, J.E., Covello, P.S., Chemistry & biology, 13(5), 2006, 513-520] http://www.sciencedirect.com/science/article/pii/S1074552106001153
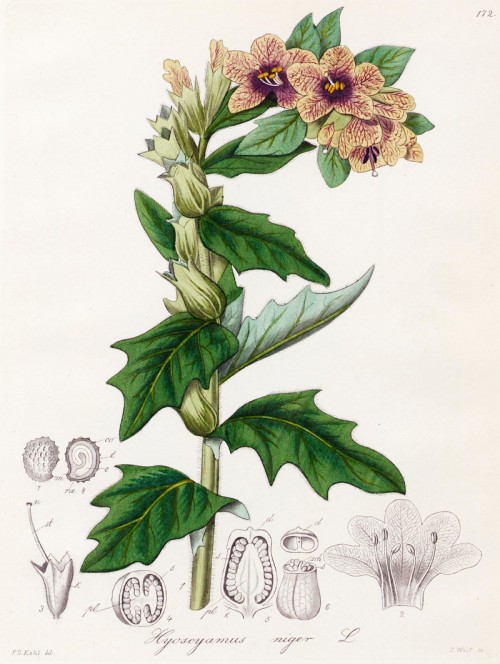
Kohl,F.G., Die officinellen Pflanzen der Pharmacopoea Germanica, t.172 (1891-1895)
http://plantgenera.org/species.php?id_species=543706

Hyoscyamus niger, CC BY-SA 3.0, Author: Andreas Kraska